Summary
Atherosclerosis is a diffuse process that starts early in childhood and progresses asymptomatically through adult life, often manifesting as coronary artery disease, stroke, transient ischemic attack, or peripheral arterial disease before the problem can be identified. Rupture of atherosclerotic plaque and its thrombotic complications are the major cause of acute coronary syndrome and cardiovascular death in the industrialized world. This article discusses some of the research that has advanced our understanding of the atherosclerotic process.
- lipid disorders genomics
- coronary artery disease
Atherosclerosis is a diffuse process that starts early in childhood and progresses asymptomatically through adult life, often manifesting as coronary artery disease, stroke, transient ischemic attack, or peripheral arterial disease before the problem can be identified. Rupture of atherosclerotic plaque and its thrombotic complications are the major cause of acute coronary syndrome (ACS) and cardiovascular (CV) death in the industrialized world. Valentin Fuster, MD, PhD, Mt. Sinai School of Medicine, New York, NY, discussed some of the research that has advanced our understanding of the atherosclerotic process.
Atherosclerotic plaques are a challenging imaging target due to their small size and tendency to change rapidly. By virtue of their enhanced spatial and temporal resolution, the new generation of imaging modalities (ie, magnetic resonance imaging, nuclear imaging, computed tomography, fluorescence imaging, intravascular ultrasonography, and optical coherence tomography) offers the possibility of identifying early atherosclerosis in at-risk individuals before the rupture occurs [Matter M et al. Eur Heart J 2009].
We now know that peripheral blood mononuclear-derived endothelial progenitor cells (EPCs) bind platelets via the CD62P receptor and inhibit platelet activation, aggregation, and adhesion to collagen and thus prevent clot formation [Abou-Saleh H et al. Circulation 2009]. We have also learned that exercise and pharmacological methods have the potential to increase and mobilize EPCs [Walther C et al. Circulation 2009] which substitutes damaged endothelial cells as a result of risk factors [Moreno PR et al. J Am Coll Cardiol 2009]. Effective and protracted reduction of low-density lipoprotein cholesterol with statins is associated with a significant (p<0.01) regression of atherosclerotic lesions [Corti R et al. Circulation 2002]. The reverse cholesterol transport (RCT) system has been shown to be the main mechanism for plaque regression [Moreno PR et al. J Am Coll Cardiol 2009]. In addition to raising high-density lipoprotein cholesterol, apolipoprotein A-I therapies and the promotion of cholesterol efflux from macrophages hold great promise and may be available for therapeutic application in the near future [Moreno PR et al. J Am Coll Cardiol 2009].
A number of independent studies now pinpoint inflammation as a key process that links multiple risk factors for atherosclerosis and its complications with altered arterial biology. Inflammasomes are multiprotein complexes that consist of caspase 1, PYCARD, a NALP, and sometimes caspase 5 or 11. Inflammasomes are responsible for activating inflammatory tissue responses. These cells are equipped with an array of signaling receptors that detect foreign molecular substances or altered endogenous molecules that appear under situations of stress [Stutz A et al. J Clin Invest 2009]. New insights into this process are coming from HIV research that show that the HIV infection may further increase cardiovascular disease risk via proatherosclerotic effects on smooth muscle cells and macrophages or by increasing inflammation [Currier JS. Top HIV Med 2009].
Death from cardiovascular disease (CVD) is and will remain for the foreseeable future the most significant cause of mortality worldwide; thus, the ability to determine an individual's risk of CVD and initiate treatment is essential. Christopher J. O'Donnell, MD, Massachusetts General Hospital and Harvard Medical School, Boston, MA, discussed some of the advances that have made it possible to estimate individual risk and initiate earlier and, in some cases, personalized treatment.
The Framingham Heart Study (FHS) is an ongoing multigenerational observational cohort study that has been used to identify CVD risk factors. Data from FHS participants were used to develop a coronary disease prediction algorithm to predict multivariate coronary heart disease (CHD) risk in patients without overt CHD [Wilson PW et al. Circulation 1998]. Recently, D'Agostino and colleagues reported on a new algorithm that expands on the Framingham CHD algorithm on the basis of a larger number of events, incorporates HDL cholesterol, and estimates absolute CVD risk [D'Agostino RB et al. Circulation 2008].
High-sensitivity C-reactive protein (hsCRP) is one of the most promising independent biomarkers for predicting future CV events. It also predicts the risk for hypertension and diabetes. Addition of hsCRP to established risk factors correctly reclassifies a substantial proportion of patients who are categorized as “intermediate-risk” by traditional risk scores into clinically relevant higher- or lower-risk categories [Ridker PM. J Am Coll Cardiol 2007]. Other biomarkers from different disease pathways, either alone or in combination, may further improve discrimination. In one study, the biomarkers that most strongly predicted major CV events were B-type natriuretic peptide level (adjusted hazard ratio, 1.25 per 1 SD increment in the log values) and urinary albumin-to-creatinine ratio (1.20) [Wang TJ et al. N Engl J Med 2006]. In another study in the elderly, the simultaneous addition of several biomarkers of CV and renal abnormalities substantially improved the risk stratification for death from CV causes beyond that of a model that was based only on established risk factors (C statistic with biomarkers vs without biomarkers, 0.766 vs 0.664; p<0.001) [Zethelius B et al. N Engl J Med 2008].
The FHS is launching a major initiative to discover risk factors and markers that could lead to new blood tests to identify individuals without known CVD who are at high risk of atherosclerosis and metabolic diseases. This initiative, the Systems Approach to Biomarker Research in Cardiovascular Disease (SABRe CVD), will identify and validate new biomarkers, such as proteins, lipids, and messenger RNA molecules that are implicated in heart disease. The study will attempt to integrate proteomic, transcriptomic, lipomic, and metabolomic information to give a more complete picture of living organisms. The term “omic discovery” is used to describe this process and refers to the comprehensive analysis of biological systems.
Data suggest that parental CVD can predict offspring events that are independent of traditional risk factors [Lloyd-Jones, et al. JAMA 2004]. The Human Genome Project has spawned several important “omic” technologies that allow “whole-genome” interrogation of sequence variation that will provide more exacting details of CVD mechanisms and, in some cases, are redefining its taxonomy. For instance, in one study, 42% of the residual variation in the quantity of coronary artery calcification was found to be attributable to genetic factors (p=0.0003) [Peyser P et al. Circulation 2002].
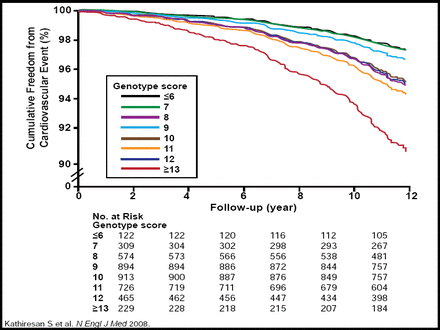
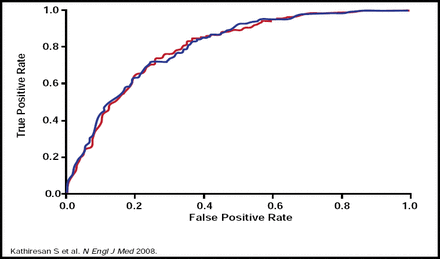
As of December 10, 2009, 443 genomewide association study papers have been published and 2083 single-nucleotide polymorphisms (SNPs) have been reported (http://www.genome.gov/26525384). Genomewide association data have identified SNPs for most of the Framingham risk factors for CVD [Kathiresan S et al. Nature Genetics 2008, 2009]. A genotype score of nine validated SNPs that are associated with modulation in levels of LDL or HDL cholesterol has been found to be an independent risk factor for CVD. The score did not improve risk discrimination but did modestly improve clinical risk reclassification for individual subjects beyond standard clinical factors (Figures 1A and 1B.) [Kathiresan S et al. N Engl J Med 2008].
Predictive Cumulative Freedom from MI, Ischemic Stroke, or Death From CHD According to Genotype Score.
Copyright © 2008 Massachusetts Medical Society. All rights reserved.
ROC Curves for Incident MI, Ischemic Stroke, or Death From CHD During 10 Year Follow-Up.
Copyright © 2008 Massachusetts Medical Society. All rights reserved.
While genome sequencing is certainly the next frontier in personalized medicine, Dr. O'Donnell concluded, we are still awaiting translation of this new information into effective prediction and prevention practices.
- © 2010 MD Conference Express