Summary
The journey from sinus rhythm toward atrial fibrillation (AF) involves several types of atrial remodeling, including (among others) electrical, contractile, and structural remodeling. This article discusses the implications of atrial remodeling on the treatment of AF, future options for antiarrhythmic therapy, and hospitalization rate among other things.
- Arrhythmias
The journey from sinus rhythm toward atrial fibrillation (AF) involves several types of atrial remodeling, including (among others) electrical, contractile, and structural remodeling. Professor Joachim R. Ehrlich, MD, Goethe-University, Frankfurt, Germany, described the implications of atrial remodeling on the treatment of AF.
Electrical remodeling refers to changes in the atrial refractory period, including shorter duration and loss of physiological rate dependence. Electrical remodeling occurs rapidly, usually within a few days, and contributes to the persistence of AF. Electrical changes arise following key changes to intracellular calcium homeostasis and calcium overload. In patients with AF, the acetylcholine-activated potassium current (IKACh), which mediates much of the cardiac response to vagal nerve stimulation, is upregulated and constitutively active. This suggests that IKACh blockers might hold therapeutic potential in AF [Ehrlich JR et al. J Am Coll Cardiol 2008].
Following electrophysiological changes, contractile remodeling occurs rapidly and creates a loss of atrial contractility, setting the stage for thrombus formation. Electrical and contractile remodeling are closely related, and recent studies suggest that selective restoration of atrial contractility with antiarrhythmic agents that block the ultrarapid delayed rectifier is possible [Schotten U et al. Cardiovasc Res 2007]. Such an intervention may hold the promise of reducing atrial stasis and eventually subsequent clot formation.
Structural remodeling leads to dilatation of the atria, alterations in cellular structure, and deterioration of normal tissue architecture. With prolonged and persistent periods of AF, cellular changes that result from structural remodeling may become irreversible. Antifibrotic agents, such as pirfenidone, have been shown to reduce arrhythmogenic atrial remodeling and vulnerability to AF in animal models [Lee KW et al. Circulation 2006], but to date the evidence in humans is lacking.
In a large meta-analysis of renin-angiotensin-aldosterone system (RAAS) blockade, treatment with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) prevented the development of AF in patients who were taking these agents for heart failure, vascular disease, and hypertension [Healy JS et al. J Am Coll Cardiol 2005]. However, the benefit was limited to patients with systolic left ventricular (LV) dysfunction or LV hypertrophy with an indication for RAAS-inhibiting medication. This suggests a role for RAAS blockers in the prevention of AF.
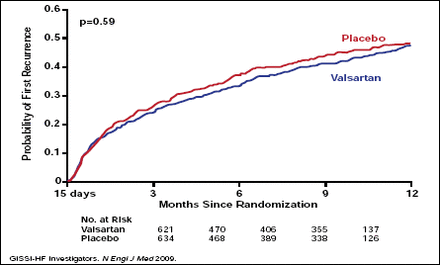
RAAS blockers may also benefit patients with lone AF in the absence of hypertension and/or heart disease. In one recent study, treatment with ramipril 5 mg daily was more effective than placebo in preventing relapses of lone AF over a follow-up period of 3 years (p<0.03) [Belluzzi F et al. J Am Coll Cardiol 2009]. However, in a broader population of patients with AF and underlying cardiovascular disease, diabetes, or left atrial enlargement, additional treatment with valsartan did not prevent AF recurrence compared with placebo (Figure 1) [GISSI-AF Investigators. N Engl J Med 2009]. These findings suggest that RAAS blockade may be less effective in the primary and secondary prevention of AF in patients with established structural heart disease, Prof. Ehrlich said.
Prospective Data – AF “All Comers.”
Copyright © 2009 Massachusetts Medical Society. All rights reserved.
Cardioversion and maintenance of sinus rhythm remain a therapeutic challenge in the setting of AF. Professor Thomas Meinertz, MD, University Heart Center, Hamburg, Germany, discussed future options for antiarrhythmic therapy in patients with AF.
The most promising of the newer antiarrhythmic therapies include atrial-selective agents and multiple channel-blocking agents. Investigational atrial-selective agents include vernakalant, AVE0118, and AZD 7009. The amiodarone congeners include dronedarone, SSR149744C, and ATI-2042. Other agents include azimilide, tedisamil, gap junction-modifying drugs (eg, rotigaptide), serotonin 5-HT4 receptor antagonists, and muscarinic M2-receptor antagonists.
Among the investigational drugs, dronedarone is the first to represent true progress in antiarrhythmic therapy. Dronedarone is structurally related to amiodarone, the most widely used antiarrhythmic in patients with AF. However, dronedarone does not contain iodine and therefore avoids the iodine-related organ toxicity that is associated with amiodarone. In the ATHENA trial, treatment with dronedarone significantly reduced cardiovascular hospitalizations and cardiovascular mortality compared with placebo in patients with AF [Hohnloser SH et al. N Engl J Med 2009]. Based in part on findings from the ATHENA trial, the U.S. Food & Drug Administration approved dronedarone in 2009 for the treatment of AF.
Hospitalization rate, one of the key outcomes of the ATHENA trial, is an important endpoint in AF studies, Prof. Meinertz said. AF is the most common form of arrhythmia that results in hospitalization. Hospitalizations for AF have risen dramatically in the past 10 years and now represent a significant economic burden for the health care community. As a result, future trials of antiarrhythmic agents should include hospitalization-related endpoints in addition to traditional clinical outcomes to measure their true impact on the overall health care system, Prof. Meinertz said.
- © 2009 MD Conference Express