Summary
Emerging technologies that address concerns that are not resolved by medication are discussed in this article, including partial circulatory support for medically refractory heart failure patients as well as a low-viscosity polymer that can be used to treat damaged tissue after acute myocardial infarction.
- Interventional Techniques & Devices Clinical Trials
- Myocardial Infarction
Emerging technologies that address concerns that are not resolved by medication were the subject of discussion at a special late-breaking clinical trial session. Two notable presentations at that session included partial circulatory support for medically refractory heart failure patients and a low-viscosity polymer that can be used to treat damaged tissue after acute myocardial infarction (AMI).
Partial Circulatory Support Improves Heart Function in Medically Refractory Heart Failure Patients
Traditional ventricular assist devices (VADs) provide full support, taking over the work of the heart's main pumping chamber in end-stage heart failure patients who are in or near cardiogenic shock. Their use is restricted, however, because they require major surgery. Daniel Burkhoff, MD, PhD, Columbia University, New York, NY, presented results from the first-in-man study of the Synergy® Pocket Micro-pump (CircuLite, Saddlebrook, NJ), a small (the size of a AA battery, weight ∼25 grams) device that can be implanted (off pump) with a mini-thoracotomy. The pump is positioned subcutaneously in a right subclavian pacemaker-like pocket. The inflow cannula inserts into the left atrium; the outflow graft connects to the right subclavian artery. The Synergy micro-pump pumps 2.5–3.0 L/min.
The primary objective of the study was to determine if partial support would be adequate to provide long-term benefits in New York Heart Association (NYHA) Class IIIb and early Class IV patients. Study endpoints included: pump performance, hemodynamic benefits, end organ function, exercise tolerance, and safety.
The study population comprised 16 subjects (13 men, 3 women; mean age 52 years) with NYHA Class IIIb (11 subjects) or early Class IV (5 subjects) heart failure despite optimal medical therapy, who were eligible for heart transplant and had a life expectancy ≥6 months without full VAD support. The average duration of support was 90 days (range 6 to 213 days).
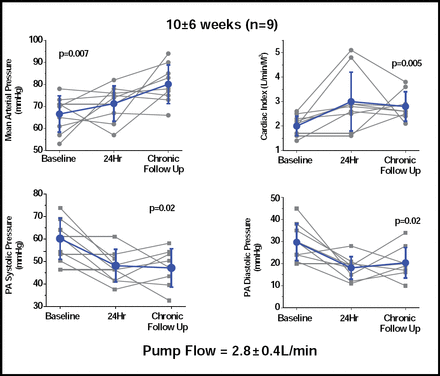
Thirteen of the 16 patients were alive at 3 months. Results for 9 patients who were followed for a mean of 10 weeks showed significant increases in mean arterial pressure, cardiac index, and systolic and diastolic pulmonary arterial pressure (Figure 1). Significant (p=0.003) reductions were also seen in capillary wedge pressure (29 ± 5 vs 17 ± 5). Adverse events (AEs) included 10 incidents of secondary thrombus in 9 patients. All occurred in patients who were using a first-generation pump, which has now been modified and tested in 11 patients for as long as 6 months without any incidence of thrombus. Other AEs included pump pocket hematoma (3 incidents), surgical bleeding, hemolysis, and sepsis (2 incidents each).
Long-term Hemodynamic Effects at 10±6 weeks (n=9).
Dr. Burkhoff concluded that the Synergy Micro-Pump has the potential to expand the use of mechanical circulatory support to a large population of patients with severe medically refractory CHF who currently are not sick enough to justify the risks that are associated with implantation of a full-support LVAD.
BL-1040 Myocardial Implant for the Treatment of Acute Myocardial Infarction
Although percutaneous coronary interventions (PCIs) and stents have done much to improve outcomes after AMI, treatment of the damaged tissue during the healing process has been lacking. Jonathan Leor, MD, Neufeld Cardiac Research Institute, Sheba Medical Center, Tel-Hashomer, Israel, presented the results of a pilot study that sought to determine the safety and efficacy of BL-1040 (BioLineRX, Jerusalem, Israel), a partially crosslinked alginate-based liquid polymer, in preventing deterioration of the damaged myocardium. BL-1040 is injected via a catheter in the infarct-related artery following PCI. It penetrates through the damaged capillaries into the damaged heart tissue, where it settles and turns into a gel that acts like a 3-dimensional scaffold and prevents thinning of the left ventricular wall. After about 6 weeks, BL-1040 is reabsorbed and excreted through the kidney.
To be eligible for the study, subjects were required to be aged between 18 and 75 years, have experienced a first AMI (with 4 of 16 akinetic segments) with successful revascularization with PCI within 7 days, and have 1 or more of the following: left ventricular ejection fraction (LVEF) >20% and <45%; peak creatine kinase >2000 IU; and infarct size >25%, as measured by MRI. The primary study outcomes included adverse events (ie, MI, ventricular arrhythmias, cardiovascular hospitalization, symptomatic heart failure, renal failure, stroke, and death). Secondary outcome measures were: change from baseline in LV dimensions, regional (infarct-related) dimensions, global wall motion score, and LVEF. Follow-up visits occurred at 30, 60, 90, and 180 days post-procedure. Of the 10 subjects who have been treated to date, 4 have completed the 6-month follow-up period and 1 patient has completed the 3-month follow-up visit. Five patients have been recruited since January 2009.
At 90 days, LVEF increased to 49% (vs 47% at baseline). Diastolic volume decreased from 132 ml to 122 ml, and LV diastolic volume decreased from 67 ml to 62 ml. Pro-brain natriuretic peptide (BNP) levels went from 830 pg/ml to 480 pg/ml. No adverse events have been reported. The investigators have concluded that the use of BL-1010 is feasible and safe. Based on these early results, the ISMB unanimously approved opening enrollment to the remaining 25 patients. The study is expected to be completed in the third quarter of 2009.
- © 2009 MD Conference Express