Summary
Approximately 50% of heart failure (HF) patients have a left ventricular ejection fraction =45%. The majority of them are women and older patients. Unlike low ejection fraction HF, the primary underlying condition in heart failure with preserved ejection fraction (HFPEF) is hypertension. There currently are no evidence-based treatments to improve outcomes in patients with HFPEF. This article presents the results of the Irbesartan in Heart Failure with Preserved Ejection Fraction Study [I-PRESERVE; NCT00095238].
- cardiology clinical trials
- heart failure
Approximately 50% of heart failure (HF) patients have a left ventricular ejection fraction (EF) ≥45%. The majority of them are women and older patients. Unlike low ejection fraction HF, the primary underlying condition in heart failure with preserved ejection fraction (HFPEF) is hypertension. There currently are no evidence-based treatments to improve outcomes in patients with HFPEF.
Peter E. Carson, MD, Georgetown University and Washington DC Veterans Affairs Medical Center, Washington, DC, presented the results of the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE; NCT00095238) at the American Heart Association Scientific Sessions meeting in New Orleans.
I-PRESERVE was a multicenter, multinational, randomized, placebo-controlled, phase 3 trial. The objective was to determine whether treatment with the angiotensin-receptor blocker irbesartan reduces all-cause mortality and hospitalizations in patients with HFPEF. A secondary objective was to better define the characteristics, natural history, and prognosis of HF in this population. The primary study endpoint was a composite of all-cause mortality or hospitalization for a cardiovascular (CV) cause. Secondary endpoints were the individual components of the primary outcome; sudden death or death or hospitalization that was related to HF; change in quality of life at 6 months (as assessed by the Minnesota Living with Heart Failure score); change in plasma NT-proBNP at 6 months; a vascular-event composite outcome that included death from CV causes, nonfatal myocardial infarction (MI), or nonfatalstroke; and death from CV causes.
The population of I-PRESERVE was comprised of 4128 subjects (mean age 72 years; 60% women) with NY Heart Association class II, III, or IV HF and an EF ≥45%. Hypertension was present in 88% of subjects and was the primary cause of HFPEF in 63%. Subjects were randomly assigned to receive a target dose of 300 mg irbesartan (n=2067) or placebo (n=2061) daily using a forced titration protocol that began with 75 mg irbesartan at Week 1 and was increased every 2 weeks as tolerated. Follow-up continued at 4-month intervals until a total of 1440 primary endpoints occurred.
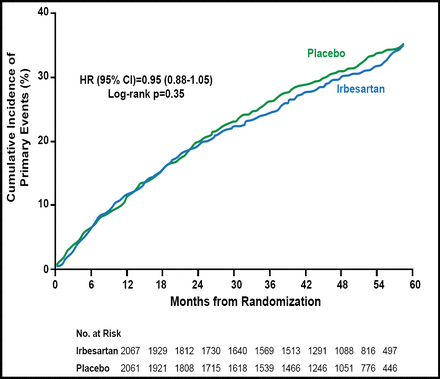
At the end of the titration phase, the mean dose of irbesartan was 275 mg/day. After a mean follow-up of 49.5 months, the primary composite endpoint occurred in 36% (742) of patients who were treated with irbesartan and 37% (763) of placebo-treated patients (HR, 0.95; 95% CI, 0.86 to 1.05; p=0.35; Figure 1). The absence of a treatment effect with irbesartan was consistent across all secondary endpoints as well as in all prespecified subgroups. Worse outcomes were seen among several prespecified subgroups, regardless of treatment, including subjects aged 75 years or older, males, subjects with EF ≤59% or diabetes, and those with an HF-related hospitalization within the prior 6 months.
Primary Endpoint: Death or Hospitalization for Heart Failure, MI, Unstable Angina, Stroke, or Arrhythmia.
Approximately 33% of subjects discontinued study participation in both treatment groups. Discontinuation of the study drug due to an adverse event was slightly more frequent in the irbesartan group compared with those who were randomized to placebo (16.1% vs 14.0%; p=0.07), although there was no significant difference in discontinuations due to serious adverse events. Patients who were randomized to irbesartan also were more likely to experience a doubling in serum creatinine (6% vs 4%; p<0.001) and an elevated potassium level >6.0 mmol/L (3% vs 2%; p=0.01).
The results of this trial were similar to those of other trials of renin-angiotensin receptor blockers in patients with HFPEF but stand in contrast to trials in patients with reduced EF. Dr. Carson commented, “In order for this field to move forward, we need a better understanding of the mechanism underlying this syndrome [HFPEF] and additional potential targets for treatment.”
The results of the I-PRESERVE study are available online at www.nejm.org .
- © 2009 MD Conference Express