Summary
This article presents a debate concerning the merits of different therapies'sulfonylureas, linides, thiazolidinediones, insulins, and incretin-based therapy'for the treatment of diabetes.
- diabetes mellitus
- prevention & screening
- endocrinology
- insulin
In this lively session, presenters debated the merits of different therapies—sulfonylureas, glinides, thiazolidinediones, insulins, and incretin-based therapy—for the treatment of diabetes.
Sulfonylureas
Leif Groop, MD, PhD, Lund University, Malmö, Sweden, defended the merits of sulfonylureas in the treatment of diabetes. Sulfonylureas, which have been in use since 1948, have well-documented efficacy as antidiabetic agents. New evidence supports the use of sulfonylureas in a broad range of patient subgroups, including patients with diabetes that is caused by mutations in the hepatocyte nuclear factor 1alpha (HNF-1alpha) gene (Pearson ER et al. Lancet 2003).
Sulfonylureas also have predictable adverse events in patients with type 2 diabetes. The incidence of severe hypoglycemia is approximately 0.8 events per 100 patient-years among those who are treated with sulfonylurea tablets, compared with 11.5 events per 100 patient-years among insulin-treated patients (Leese GP et al. Diabetes Care 2003).
As with other available antidiabetic treatments, the sulfonylureas and glinides do not change the course of the disease, Prof. Groop said. Regardless of the choice of therapy, the initial benefits of glycemic control are lost over time. In the UK Prospective Diabetes Study (UKPDS; ISRCTN75451837), HbA1c levels rose among patients who were treated with conventional strategies (primarily diet alone), glibenclamide, chlorpropamide, metformin, or insulin (UKPDS. Lancet 1998).
Given the inevitable decline in efficacy, treatment cost becomes particularly important, Prof. Groop argued. He estimated that sulfonylureas are 10 times less expensive than other therapies, including insulin, the glitazones, and protease dipeptidyl peptidase (DPP)-IV inhibitors.
“No durability has been demonstrated for any of the treatments discussed,” Prof. Groop said. “When choosing from a group of poor performers, take the least expensive agent.” This approach can only lead to the selection of sulfonylureas as adjunct therapy to metformin, he concluded.
Thiazolidinediones
Richard W. Nesto, MD, Harvard Medical School, Boston, MA, defended the use of thiazolidinediones in the treatment for hyperglycemia. As a cardiologist, Dr. Nesto said he was particularly interested in the cardiovascular benefits that are associated with thiazolidinediones.
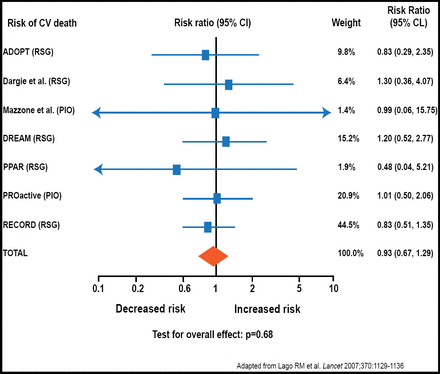
In 2007, Nissen et al. raised concerns that rosiglitazone increased the risk of cardiovascular death (Nissen SE et al. N Engl J Med. 2007). “This is a myth,” Dr. Nesto said. Several careful analyses have shown that rosiglitazone does not exacerbate cardiovascular risk in patients with type 2 diabetes (Figure 1). In fact, thiazolidinediones improve several cardiovascular risk factors. For example, thiazolidinediones reduce blood pressure by an average of 3.5/1.8 mm Hg. Thiazolidinediones also modify waist-hip ratio, high-density lipoprotein cholesterol (HDL-C), albuminuria, and the presence of inflammatory biomarkers, such as C-reactive protein (CRP).
Rosiglitazone Does Not Exacerbate Cardiovascular Risk.
In major cardiovascular trials, thiazolidinediones have been associated with clinically relevant reductions in atherosclerosis, as measured by carotid intima-media thickness (CIMT) and intravascular ultrasound (IVUS). Thiazolidinediones also have reduced the need for revascularization following percutaneous coronary intervention (PCI).
“Thiazolidinediones are the only drug class with a cardiovascular benefit,” Dr. Nesto said. With careful patient selection, thiazolidinediones can provide effective glycemic control while reducing a range of cardiovascular risk factors. Therefore, thiazolidinediones are the best choice for the comprehensive care of patients with type 2 diabetes, he said.
Insulins
Hannele Yki-Järvinen, MD, University of Helsinki, Helsinki, Finland, described strategies to maximize the benefits of insulin therapy. In particular, she noted that the recent Action to Control Cardiovascular Risk in Diabetes (ACCORD; NCT00000620) trial illustrated the important balance between the benefit and risk that are associated with intensive glucose control.
In ACCORD, in intensively treated patients who achieved an HbA1c of 6.4%, there was a notable increase in the risk of cardiovascular deaths, Prof. Yki-Järvinen said. Therefore, based on the UKPDS, she suggested that when drugs are used to treat type 2 diabetes, therapeutic glycemic targets should not be lower than 7.0%.
With the variety of options for reducing HbA1c levels, some physicians may be concerned about the ability of insulin to provide adequate glycemic control, Prof. Yki-Järvinen said. However, the HbA1c target of 7.0% has been reached in several recent trials that have incorporated aggressive insulin titration.
Weight gain and hypoglycemia are important potential drawbacks to insulin therapy. Although the HbA1c target of 7.0% can be reached with a variety of insulin-based regimens, analysis of both old and recent evidence in the literature has shown that mixed and prandial/multiple injections increase weight gain and the incidence of hypoglycemia, compared with regimens that use only basal insulin, regardless of the use of oral hypoglycemic agents. In addition, use of basal insulin analogs reduces hypoglycemia while offering similar glycemic control compared with neutral protamine hagedorn (NPH) insulin.
In summary, Prof. Yki-Järvinen argued that insulin is an effective option for glycemic control in appropriate patients with type 2 diabetes, especially the use of aggressively titrated basal insulin analogs that are combined with oral agents.
Incretin-Based Therapy
Michael Nauck, MD, PhD, Diabeteszentrum Bad Lauterberg, Bad Lauterberg, Germany, provided a comprehensive review of the strengths and drawbacks of major antidiabetic therapies. In particular, he focused on therapies that are used after metformin fails.
Prof. Nauck developed a scoring system to rank therapies on a number of criteria, assigning points for each of the following parameters:
-
efficacy with regard to glycemic control
-
prevention of microvascular complications
-
prevention of macrovascular complications
-
attractive mechanism of action
-
potential for serious adverse events
-
potential for unpleasant side effects
-
proven cardiovascular safety
-
effects on body weight
-
potential to cause hypoglycemia
-
need for glucose self-monitoring
-
potential for durability of glycemic control
-
drug costs per day
Each parameter received a numerical value that ranged from −2 (very negative) to +2 (very positive). Using this system, Prof. Nauck calculated scores for 5 therapeutic groups: sulfonylureas/glinides; thiazolidinediones; insulin; incretin mimetics; and DPP-IV inhibitors (a subtype of incretin therapy).
In Prof. Nauck's analysis, sulfonylureas/glinides received the lowest score (total score, −1), suggesting that they were the least favorable therapeutic option (Table 1). Scoring only slightly higher, insulin (total score, +1) and thiazolidinediones (total score, +1.5) were the next most desirable therapies.
Ranking of Second-Line Therapies in Type 2 Diabetes.
Incretins (total score, +6) and DPP-IV inhibitors (total score, +7) scored markedly higher than their older therapeutic counterparts. With the exception of cost, DDP-IV inhibitors were the only drug class to receive no negative scores on the individual parameters of Prof. Nauck's scoring system. Incretins received negative scores for the potential for serious or unpleasant side effects and for cost.
Based on his comparison of available agents, Prof. Nauck said that incretin-based therapies, including DPP-IV inhibitors, could become the preferred second-line therapy for patients who have not achieved optimal glycemic control despite lifestyle interventions and metformin therapy.
- © 2008 MD Conference Express