Summary
Results from the 3T/2R study [NCT00398463] demonstrated that the addition of tirofiban to standard therapy decreased the rate of myocardial infarction and resulted in a lower rate of major cardiovascular events 30 days after percutaneous coronary intervention compared with standard antiplatelet therapy alone in poor responders to aspirin or clopidogrel.
- myocardial infarction
- interventional techniques & devices clinical trials
Results from the 3T/2R study (NCT00398463) demonstrated that the addition of tirofiban to standard therapy decreased the rate of myocardial infarction (MI) and resulted in a lower rate of major cardiovascular events (MACE) 30 days after percutaneous coronary intervention (PCI) compared with standard antiplatelet therapy alone in poor responders to aspirin or clopidogrel.
Individual response to aspirin or clopidogrel varies considerably among patients. Prior studies have shown that patients who have a poor response to or are resistant to one or both of these medications are at higher risk for subsequent cardiovascular events, particularly after PCI. Adjunctive inhibition of platelet glycoprotein (GP) IIb/IIIa receptors has been shown to reduce the overall risk of death or nonfatal MI 30 days after PCI [Topol EJ et al. N Engl J Med 2001]. Tirofiban is a highly selective, short-acting inhibitor of fibrinogen binding to platelet GP IIb/IIIa that inhibits ex vivo platelet aggregation in response to a variety of agonists.
The main purpose of this study was to assess, in poor responders to oral antiplatelet therapy, whether tirofiban in addition to standard antiplatelet therapy can reduce the incidence of MI after elective coronary angioplasty compared with standard therapy alone.
This was a randomized, double-blind, multicenter, placebo-controlled, proof-of-concept study in patients aged 18 to 75 years who were scheduled to undergo elective PCI for silent ischemia, stable angina, or low-risk non-ST-segment elevation acute coronary syndrome who were determined (using VerifyNow™ Aspirin and P2Y12 point-of-care assays) to be poor responders to aspirin or clopidogrel. Aspirin poor response was defined as aspirin reaction units (ARU) >550, and clopidogrel resistance as <40% platelet inhibition. The primary study endpoint was troponin I/T elevation >3X ULN in one or more blood samples within 48 hours after PCI. Secondary endpoints included CK-MB mass elevation >1X, 3X, or 5X ULN; MACE; or stent thrombosis based on ARC classification. All patients received heparin or bivalirudin, aspirin, and 300 mg (6 hours before PCI) or 600 mg (2 hours before PCI) clopidogrel. Patients in the tirofiban arm (n=132) also received tirofiban 25 μg/kg (high-bolus dose regimen), administered as a 3-minute bolus followed by a 14 to 24-hour 0.15-μg/kg/min infusion. A full discussion of the rationale and study protocol has already been published [Valgimigli M et al. Cardiovasc Drugs Ther 2008].
A total of 263 of 1277 patients who were scheduled for elective PCI met criteria for inclusion in the study. Within 48 hours after PCI, troponin I/T values >3X ULN were found in 35.1% of patients who were treated with standard care versus 20.4% of the patients who were treated with standard care plus tirofiban (relative risk reduction [RRR] 42%; 95% CI, 12 to 61; p=0.009). Similar benefit for add-on treatment with tirofiban was seen in both aspirin and clopidogrel poor responders separately.
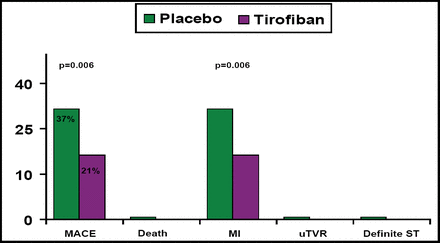
At 30 days, the MACE rate was significantly lower in patients who were treated with add-on tirofiban (21%) versus those who were treated with standard care only (37%; p=0.006), with the difference being driven almost entirely by the rate of MI (Figure 1).
30-Day Outcomes Efficacy Endpoints.
CK-MB levels were lower in patients who were treated with add-on tirofiban but were significantly different from placebo only for those who had levels >1X ULN (RRR 62%; p=0.001). Safety results were similar in both groups. There was no major bleeding. The rate of minor bleeding was very low (p=0.99) and did not differ between the two groups.
A recently published study [Bonello L. J Am Coll Cardiol 2008] demonstrated that administration of additional clopidogrel (600 mg every 24 hours for 1 to 3 doses), guided by the vasodilator-stimulated phosphoprotein index, is an effective method to reduce MACE following PCI in patients with clopidogrel resistance. Further studies are needed to compare these 2 successful strategies (additional clopidogrel vs high-bolus dose tirofiban) to manage patients who have a poor response to antiplatelet therapy who are undergoing PCI. Given the difference in timing that is required to achieve an adequate antiplatelet effect, consideration of the clinical circumstances may dictate which strategy is preferred.
- © 2008 MD Conference Express