Summary
In the last year, there has been an explosion in the discovery of new genes that are related to autoimmunity. The sudden increase began with the discovery of a single nucleotide polymorphism in the gene PTPN22 that holds a 2-fold increased risk for a number of autoimmune disorders, including rheumatoid arthritis (RA). This article discusses the significant variations in risk alleles, sometimes based on ethnic groups, and how these variations confer significant differences in risk for RA diseases.
- inflammatory disorders
- rheumatoid arthritis
- lupus
New Genes in Rheumatoid Arthritis (RA)
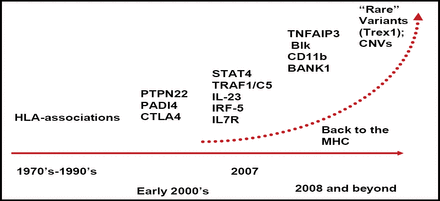
In the last year, there has been an explosion in the discovery of new genes that are related to autoimmunity (Figure 1). The sudden increase began with the discovery of a single nucleotide polymorphism (SNP) in the gene PTPN22 that holds a 2-fold increased risk for a number of autoimmune disorders, including RA. Peter Gregersen, MD, Feinstein Institute for Medical Research, Manhasset, NY, discussed the significant variations in risk alleles, sometimes based on ethnic groups, and how these variations confer significant differences in risk for RA diseases.
Accelerating Discovery of Genes for Autoimmunity.
Two new gene areas of interest are the TRAF1 (Figure 1) and STAT4 loci. Genetic variants at the TRAF1-C5 locus on chromosome 9 are associated with an increased risk of anti-CCP-positive RA and chronic inflammation [Plenge RM et al. N Engl J Med 2007], and significantly (p=0.008) increased radiographic progression [Kurreeman FAS et al. PLoS Med 2007]. A SNP haplotype in the third intron of STAT4 is associated with susceptibility to both RA and systemic lupus erythematosus (SLE), suggesting a shared pathway for these illnesses. The odds ratio for having the risk allele in chromosomes of RA patients versus controls was 1.32 [Remmers EF et al. N Engl J Med 2007]. Beyond these new discoveries, Dr. Gregersen added, there are multiple independent genetic risk factors in the large genomic region that is known as the major histocompatibility complex (MHC) that impact RA.
New Genes in Ankylosing Spondylitis (AS)
Matthew Brown, MD, University of Queensland, Brisbane, Australia, discussed the of progress that has been made in identifying genes that are involved in the risk of developing AS with the advent of high-density linkage disequilibrium mapping. The technique has identified 2 genes so far, IL23R and ARTS1, which have a combined population attributable risk >30% [Brown MA. Rheumatology 2007]. Along with the previously identified HLA-B27 gene, these genes can predict the risk of AS in 70% of cases. The IL23R gene encodes a cytokine receptor on a subset of effector T-cells. Variants of IL23R have been identified in patients with Crohn disease and those with inflammatory bowel disease, as well as AS, suggesting a commonality for these pathologies. The ARTS1 gene encodes a protein that is involved both in immune regulation and in the processing of cell surface receptors for proinflammatory cytokines. “The successful identification of ARTS1 and IL23R should give those who are involved in AS genetics research great encouragement of the potential of this research,” Prof. Brown concluded.
New Genes in Systemic Lupus Erythematosus (SLE)
Timothy J. Vyse, MD, PhD, Imperial College of London, London, UK, reviewed recent data from genome-wide association studies (GWAS) that identified genes that are associated with an increased risk for SLE. There is overwhelming evidence that the MHC is the primary locus for these genes. Within the MHC, he identified the ITGAM, BANK1, LYN, BLK and ATG5, MECP2, UBE2LS, and SCUBE1 genes as being significant risk factors for SLE. Despite the number of new SLE associated genes that already has been identified, Prof. Vyse believes there are more to be discovered.
Quantitative Heritability of ACPA-Positive and ACPA-Negative Rheumatoid Arthritis
Diane van der Woude, MD, Leiden University Medical Center, Leiden, The Netherlands, presented the results of a study that investigated the quantitative contribution of genetic factors to disease development: the heritability. In a cohort of subjects that consisted of 148 twins in which at least one twin had RA, the overall heritability of RA was 66% (95% CI, 44–75). For anti-citrullinated protein antibody (ACPA)-positive RA, heritability was 68% (95% CI, 55–79), compared with 66% (95% CI, 38–83) for ACPA-negative RA. She concluded that these 2 subsets of RA have comparable heritability. This indicates that there may be many genetic risk factors for ACPA-negative RA that remain to be discovered.
- © 2008 MD Conference Express