Summary
For patients with acute ST-elevation myocardial infarction (STEMI) who undergo primary coronary intervention, the addition of abciximab does not enhance the clinical benefits that are provided by pretreatment with high-dose clopidogrel, according to the findings of the Bavarian Reperfusion Alternatives Evaluation-3 [BRAVE-3] trial. Moreover, abciximab increases the risk of adverse clinical outcomes, suggesting that it should not be added to standard antiplatelet therapy in this patient population.
- interventional techniques & devices
- myocardial infarction clinical trials
For patients with acute ST-elevation myocardial infarction (STEMI) who undergo primary coronary intervention (PCI), the addition of abciximab does not enhance the clinical benefits that are provided by pretreatment with high-dose clopidogrel, according to the findings of the Bavarian Reperfusion Alternatives Evaluation-3 (BRAVE-3) trial. Moreover, abciximab increases the risk of adverse clinical outcomes, suggesting that it should not be added to standard antiplatelet therapy in this patient population.
“Therapy without abciximab would certainly be more cost-effective and reduce the risk of bleeding complications,” said lead author Julinda Mehilli, MD, Deutsches Herzzentrum, Technical University, Munich, Germany.
Prior studies in primary PCI suggested that antiplatelet therapy with intravenous glycoprotein IIb/IIIa inhibitors improved clinical outcomes in patients with acute STEMI, Dr. Mehilli said. Given the trend toward greater benefit with more robust platelet inhibition, BRAVE-3 was designed to evaluate whether the intravenous glycoprotein inhibitor abciximab reduces infarct size in patients with acute STEMI undergoing PCI following pretreatment with 600 mg clopidogrel, a dose that is higher than currently recommended in STEMI treatment guidelines.
BRAVE-3 enrolled 800 patients with acute STEMI who presented within 24 hours of symptom onset. After pretreatment with clopidogrel (600 mg), aspirin (500 mg), and unfractionated heparin (UFH) (5000 IU), patients were randomly assigned to therapy with abciximab (n=401) or placebo (n=399). Following PCI, all patients received additional treatment with clopidogrel 75 mg twice daily for 3 days, clopidogrel 75 mg once daily for at least 4 weeks, and aspirin 200 mg once daily indefinitely.
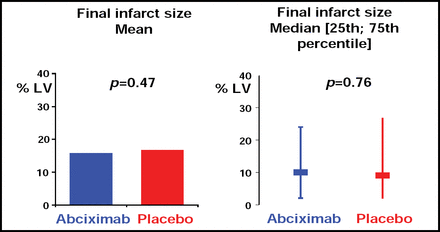
Abciximab did not provide additional reduction of the infarct size (the primary endpoint), which was expressed as a percentage of the left ventricle and measured 5–7 days after randomization. The final mean infarct size was similar in patients treated with abciximab and placebo (15.7% vs 16.6%, respectively; p=0.47; Figure 1).
Infarct Size Following Primary PCI With and Without Abciximab.
In the first 30 days following PCI, abciximab did not increase the rate of TIMI major bleeding compared with placebo (1.8% in both groups). However, abciximab did increase the rates of TIMI minor bleeding and thrombocytopenia
In summary, these findings suggest that abciximab on a background of 600 mg clopidogrel did not reduce infarct size in patients with STEMI undergoing primary PCI.
- © 2008 MD Conference Express