Summary
Bivalirudin failed to show a superior “net clinical benefit” compared with unfractionated heparin (UFH) in patients undergoing percutaneous coronary intervention in the Intracoronary Stenting and Antithrombotic Regimen-Rapid Early Action for Coronary Treatment 3 [ISAR-REACT 3] trial. However, bivalirudin reduced the rate of major and minor bleeding events in this patient population.
- interventional techniques & devices clinical trials
- thrombotic disorders
Bivalirudin failed to show a superior “net clinical benefit” compared with unfractionated heparin (UFH) in patients undergoing percutaneous coronary intervention (PCI) in the Intracoronary Stenting and Antithrombotic Regimen-Rapid Early Action for Coronary Treatment 3 (ISAR-REACT 3) trial. However, bivalirudin reduced the rate of major and minor bleeding events in this patient population.
In the ISAR-REACT 3 trial, 4570 patients with biomarker-negative stable or unstable angina were randomly assigned to treatment with bivalirudin (n=2289) or UFH (n=2281) prior to scheduled PCI. All patients received clopidogrel 600 mg at least 2 hours before PCI and at least 325 mg oral or intravenous aspirin. Bivalirudin was administered as a 0.75 mg/kg loading dose followed by 1.75 mg/kg/hr infusion. Patients in the UFH group received a 140 U/kg bolus followed by placebo infusion.
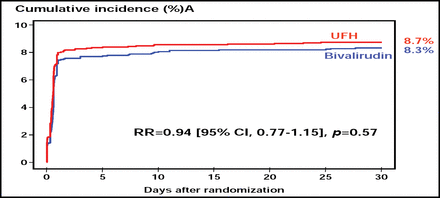
Following PCI, patients received clopidogrel 75–150 mg/day until discharge (≤3 days) and 75 mg/day for at least 1 month after balloon angioplasty or implantation of bare-metal stents and for at least 6 months after implantation of drug-eluting stents. All patients received aspirin 80–325 mg/day indefinitely. At 30 days, the cumulative incidence of death, myocardial infarction, urgent target vessel revascularization, and major bleeding (the primary endpoint) in the bivalirudin and UFH groups was 8.3% and 8.7%, respectively (RR 0.94; p=0.57; Figure 1).
Net Clinical Benefit of Bivalirudin and UFH.
Despite similarities in net clinical benefit, bivalirudin showed a clear advantage over 140 U/kg UFH with regard to risk of bleeding. Compared with those in the UFH group, bivalirudin-treated patients had a significantly lower rate of major bleeding (3.1% vs 4.6%; p=0.008) and minor bleeding (6.8% vs 9.9%; p=0.0001).
“Bleeding is important to patient outcomes,” said Harvey White, MD, Auckland City Hospital, Auckland, New Zealand. “Strikingly, bivalirudin reduced TIMI major bleeding by 50 percent.”
Still, “ISAR-REACT 3 was powered for its primary endpoint, so conclusions should focus on the primary endpoint,” said lead study author Adnan Kastrati, MD, Technische Universität, Munich, Germany. Dr. Kastrati cautioned that because the dose of UFH (140 U/kg) was higher in ISAR-REACT 3 than that used in other recent PCI trials and the dose (70–100 U/kg) recommended in current PCI guidelines in the absence of GP IIb/IIIa inhibitors [Smith.Circulation 2006], it was not clear how much of an effect the higher UFH dose had on the observed ischemic or bleeding event rates in the trial.
Dr. White noted that the ISAR-REACT 3 results should not be generalized to patients with higher ischemic risk, such as those with elevated troponin levels, acute MI, or recent CABG. Furthermore, the findings are not applicable to patients who have not received 600 mg clopidogrel ≥2 hours before PCI, he concluded.
- © 2008 MD Conference Express