Summary
In this article the primary investigator in the ENHANCE study presents the study results to address the controversy surrounding its results. ENHANCE was performed in patients with heterozygous familial hypercholesterolemia (HeFH), a genetic mutation affecting the LDL receptor that occurs in 0.2% of the population and results in average LDLs >300 mg/dL. Unlike these prior trials, ENHANCE evaluated whether ezetimibe (a selective inhibitor of cholesterol absorption in the small intestine), when added to simvastatin, reduced the progression of atherosclerosis compared with simvastatin alone in patients with HeFH.
- cardiology clinical trials
- lipid disorders
Because of the controversy surrounding the results of the ENHANCE trial, the American College of Cardiology set aside a special session during which John Kastelein, MD, PhD, Academic Medical Center, Amsterdam, The Netherlands, the primary investigator in the ENHANCE study, presented the study results which was followed by a panel discussion.
ENHANCE was performed in patients with heterozygous familial hypercholesterolemia (HeFH), a genetic mutation affecting the LDL receptor that occurs in 0.2% of the population and results in average LDLs >300 mg/dL. The primary study outcome was the change from baseline in the mean carotid artery intima-media thickness (cIMT) as measured using B-mode ultrasonographic imaging. Previous studies in patients with HeFH with pravastatin [Wiegman A et al. JAMA 2004] and atorvastatin [Smilde TJ et al. Lancet 2001] had demonstrated a tendency toward regression of atherosclerosis as determined by serial measurements of cIMT with these statins. Unlike these prior trials, ENHANCE evaluated whether ezetimibe (a selective inhibitor of cholesterol absorption in the small intestine), when added to simvastatin, reduced the progression of atherosclerosis compared with simvastatin alone in patients with HeFH.
The study population of ENHANCE consisted of men and women aged 30–75 years with HeFH and a baseline LDL-C level >210 mg/dL (either untreated or after a 6-week washout period if on prior statin therapy). Subjects were randomly assigned to receive either ezetimibe 10 mg plus simvastatin 80 mg (n=357) or simvastatin 80 mg alone (n=363). Over the 2-year course of the trial, 7 cIMT assessments were conducted (2 at baseline, one each at 6, 12, and 18 months, and 2 at Month 24). The baseline characteristics of the 2 treatment groups were similar to those seen in prior studies, with the exception of a history of myocardial infarction (MI), which was significantly lower than in previous studies.
There was no difference in the primary outcome (mean change in the cIMT defined as the average of the means of the far wall intima-media thickness of both common carotids, bulbs, and internal carotids) between the simvastatin-only and the combined therapy group (0.0058 vs 0.0111, p=0.29). No differences between treatment groups were observed in the various secondary outcomes that assessed the intima-media thickness of different portions of the carotid artery or the femoral artery.
Despite the lack of a difference in intima-media thickness between treatment groups, the addition of ezetimibe to simvastatin treatment did result in a 16.5% incremental reduction (51.4 mg/dL absolute difference) in LDL-C level over 2 years (p<0.01). Significant improvements were also seen for other lipids and apolipoproteins, with the exception of Apo A1 (Table 1). There was also a 26% incremental reduction in hsCRP (p<0.01) with combined therapy.
Percentage Changes from Baseline in Lipids, Apolipoproteins, and C-Reactive Protein.
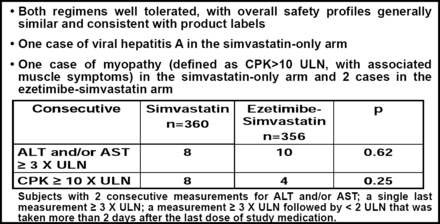
With respect to the safety of ezetimibe, Prof. Kastelein indicated that despite the reports in the press, the data demonstrated no evidence of liver or muscle toxicity (Figure 1).
Safety Observations.
The number of patients who experienced any adverse event (29.5% vs 34.2%) and who discontinued therapy due to adverse events (9.4% vs 8.1%) were similar between the simvastatin-only and combined therapy groups (p=NS for both comparisons). Cardiovascular events (cardiovascular death, nonfatal MI, non-fatal stroke, coronary revascularizations) were infrequent (2.4%) and similar (7 events simvastatin-only, 10 events combined therapy) between the two groups.
Prof. Kastelein offered several possible explanations why the combination of ezetimibe + simvastatin did not reduce cIMT compared with simvastatin only, despite achieving a more favorable lipid profile and greater incremental reduction in C-reactive protein:
-
The measurement technique was not sufficiently accurate to detect changes in atherosclerotic burden
-
Ezetimibe lacked vascular benefit despite the greater incremental changes in LDL-C and hsCRP
-
The population studied was at too low a risk to detect changes in cIMT at 2 years
Dr. Kastelein did not believe that a lack of precision in the measurement technique explained the absence of a difference between treatment groups, because measures of quality for the cIMT measurement (eg, intraclass correlation coefficient, standard deviations) exceeded the current standards that were previously shown to be necessary for accuracy. Turning to the compound itself, Prof. Kastelein reviewed the results of a prior study, which showed that simvastatin treatment increased the number of functionally active endothelial progenitor cells, whereas ezetimibe did not. [Landmesser U et al. Circulation 2005]. “It is possible that there might be something [happening] with statin therapy that is over and above simply LDL-lowering,” he said. “However, this goes against other data, which indicate that it is LDL-lowering that is important, not how you lower it.” [Robinson JG et al. J Am Col Cardiology 2005]. Dr. Kastelein thinks that the highly treated study population is the most likely reason that ENHANCE did not reach its endpoints. He pointed out that over the last 10–15 years, intensive treatment has shifted the cIMT distribution of patients with familial hypercholesterolemia from very abnormal to less abnormal, predominantly due to intensive lipid-lowering with high-dose statins +/- additional drugs. More than 80% of the patients in ENHANCE had been receiving statin therapy before enrollment, resulting in a baseline cIMT of only 0.70 mm, thus making it very difficult to show an effect with the addition of any other therapy.
Following Prof. Kastelein's presentation, there was a panel discussion during which the discussants attempted to put the study results into the context of clinical practice.
Patrick T. O'Gara, MD, Brigham and Women's Hospital, Boston, MA, began by reminding the audience that “ENHANCE was a surrogate endpoint trial in a select patient population that used the change in cIMT as a primary outcome measure. The trial is not a referendum on the established health benefits of LDL-cholesterol-lowering, especially in our high-risk patients and especially with statin medications.” Dr. O'Gara concluded by noting that the ENHANCE trial results “have increased interest in the 3 ongoing clinical endpoint trials with simvastatin and ezetimibe, most notably the IMPROVE-IT study, which has enrolled ∼11,000 of the targeted 18,000 post-ACS patients.”
Harlan M. Krumholz, MD, Yale University, New Haven, CT, told the audience that the results of the ENHANCE trials should “…change practice”—especially in the way we [in the United States] have been prescribing ezetimibe. According to Dr. Krumholz, this study “…provides no new evidence to support the use of ezetimibe and it moves us toward more uncertainty about the benefits of the drug.” He also stated, however, that whether the incremental lowering of LDL by this drug would have an effect on the progression of atherosclerosis is worthy of study, as is whether it would have an effect on patient outcomes. Dr. Krumholz pointed out that although LDL is an important risk factor for cardiovascular disease, studies (eg, hormone replacement therapy and torcetrapib) have already refuted the assumption that just because a drug lowers LDL, we can know what effect it is going to have on our patients.
The controversy over the results of the ENHANCE trial will likely remain until the results of the IMPROVE-IT trial are available in 2012. In the meantime, the American Heart Association and the American College of Cardiology have issued the following joint statement on ENHANCE:
‘The study reinforces the need to adhere to current American College of Cardiology/American Heart Association Guidelines which recommend statins to the maximally tolerated dose or to goal as first line treatment for patients with coronary artery disease. The data from the ENHANCE study should be considered as the NHLBI guidelines writing group is working on their update of the national cholesterol treatment guidelines in the coming months.’
Full text of the statement is available at: http://americanheart.mediaroom.com/index.php?s=43&item=386
- © 2008 MD Conference Express