Summary
Long-term use of celecoxib is associated with an increase in cardiovascular (CV) risk for some patients, according to findings from a pooled analysis of 6 placebo-controlled, randomized clinical trials. The degree of risk is associated with both baseline CV risk and celecoxib dose.
- cardiology clinical trials
- prevention & screening
- arthritis
Long-term use of celecoxib is associated with an increase in cardiovascular (CV) risk for some patients, according to findings from a pooled analysis of 6 placebo-controlled, randomized clinical trials. The degree of risk is associated with both baseline CV risk and celecoxib dose.
“These data should provide comfort in prescribing celecoxib to patients with very low [baseline] cardiovascular risk,” said Scott D. Solomon, MD, Brigham and Women's Hospital, Boston, MA. “Similarly, we should be cautious in prescribing celecoxib to patients who have elevated baseline risk,” he said.
Conducted in partnership with the National Cancer Institute, the Cross Trial Safety Analysis was designed to characterize the long-term CV risk associated with celecoxib, a cyclooxygenase-2 (COX-2) inhibitor. Results of the analysis were published immediately following the late breaking trials session in an online version of the journal Circulation [Solomon et al. Circulation 2008].
Together, the 6 participating trials enrolled 7950 patients and provided the equivalent of 16,070 years of patient follow-up for analysis. Patients with no CV risk factors at baseline were described as low-risk for future cardiac events. Moderate-risk patients had at least one of the following risk factors: age >75 years, hypertension, hyperlipidemia, current tobacco use, or concurrent use of low-dose aspirin. Patients with 2 or more risk factors, and those with diabetes or previous CV disease, were classified as high-risk.
Across all patient groups (median follow-up of 31 months) and dosing regimens, the risk of CV events was elevated with celecoxib use compared with placebo [HR 1.6; 95% CI, 1.1–2.3; p=0.034].
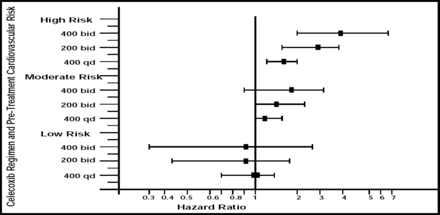
The risk of CV events varied across the different dosing regimens that were evaluated in the trial (p value for dose regimen effect = 0.0005). Hazard ratios for the primary endpoint—a composite of CV death, myocardial infarction, stroke, heart failure, or thromboembolic event—were 1.1, 95% CI, 0.6–2.0 for the once-daily 400 mg dose, 1.8, 95% CI, 1.1–3.1 for the 200 mg dose given twice a day, and 3.1, 95% CI, 1.5–6.1 for the twice-daily 400 mg dose.
Dr. Solomon and colleagues also observed an interaction between baseline CV risk and celecoxib regimen (interaction p=0.034). For patients with the lowest baseline CV risk, the risks associated with the 400 mg once daily, 200 mg twice daily, and 400 mg twice daily regimens were roughly equivalent, with hazard ratios of 1.0, 0.9, and 0.9, respectively. However, for patients with the highest baseline CV risk, the differences across doses were pronounced. The hazard ratios for the primary endpoint were 1.5, 95% CI, 1.2–1.9, 2.3, 95% CI, 1.5–3.4, and 3.5, 95% CI, 1.9–6.4, respectively (Figure 1).
Risk of Celecoxib-Related CV Events by Celecoxib Dose and Baseline CV Risk.
- © 2008 MD Conference Express