Summary
This article discusses peroxisome proliferator-activated receptors (PPARs) in an overview of the current knowledge concerning the effects of PPAR agonists on the vascular bed.
- ischemia
- cerebrovascular disease genomics
- cerebrovascular disease
Eyal Leibovitz, MD, McGill University, Montreal, Quebec, Canada, gave a brief tutorial on PPARs (peroxisome proliferator-activated receptors) and presented an overview of the current knowledge concerning the effects of PPAR agonists on the vascular bed.
Peroxisomes are intracellular organelles that are predominant in nearly all mammalian cells. PPARs received their name when it was shown that the activation of these receptors was associated with proliferation of peroxisomes in rodents. There are 3 types of PPARs: PPARα, PPARγ, and PPARβ. The natural activators of the PPARs are fatty acids, but some prostaglandins and leukotrienes also activate them, and they can also be activated by synthetic compounds, including fibrates, which activate PPARα and are an accepted treatment for dyslipidemia, and the thiazolidinediones (TZDs), which activate PPARγ and are an accepted treatment for type 2 diabetes mellitus. PPARβ/δ activators are experimental and are not used clinically.
Both PPARα and γ are expressed in human vascular endothelial cells [Delerive P et al. Circ Res 1999], and animal studies have shown that PPARα and γ may exert vascular protective effects in hypertension and other forms of cardiovascular disease by interfering with signaling pathways that lead to endothelial dysfunction, vascular remodeling, inflammation, oxidative stress, and the growth and progression of atherosclerosis [Diep et al. Circulation 2002; Diep et al. Hypertension 2002; Collins AR et al. ATVB 2001].
Clinical evidence regarding the vascular effects of PPAR activators come from studies of dyslipidemia (PPARα activators) and diabetes mellitus (PPARγ activators). The use of fibrates has been clearly shown to reduce morbidity and mortality among diabetic dyslipidemic patients; however, the results among nondiabetic dyslipidemic individuals, especially those with low HDL levels, are not as clear. The results with PPARγ activators, although effective in controlling metabolic aspects of diabetes, remain controversial from the point of view of cardiovascular protection.
Although the use of selective PPARγ activators may exert vascular protective effects in hypertension or other forms of cardiovascular disease, Dr. Leibovitz concluded with a caution that one of the PPARγ agonists has been associated with a significant increase in the risk of myocardial infarction and heart failure, and with an important but not statistically significant increase in the risk of death from cardiovascular causes [Nissen SE, Wolski K. NEJM 2007; Nissen SE et al. JAMA 2005].
Frank M. Faraci, PhD, University of Iowa, Iowa City, IA, and his colleagues have been studying mice that have been genetically altered to express dominant-negative mutations of the human PPARγ gene (PPARγ P465L, for example). In these “humanized” mice the subsequent interference with PPARγ signaling caused selective endothelial dysfunction in both cerebral arteries and arterioles. The impact of PPARγ interference was prominent in the cerebral circulation and the aorta was relatively normal in these mice.
Levels of superoxide (an oxygen-derived free radical) were increased in cerebral arterioles, and impaired endothelial function could be restored to normal with a scavenger of superoxide in PPARγ P465L mice, suggesting that mechanisms that account for impairment of cerebral vascular function following interference with PPARγ involve oxidative stress.
The implications of these findings extend far beyond regulation of vascular tone, because the endothelium affects vascular structure, blood cells, and neuronal function. Other studies that have been conducted by Dr. Faraci and colleagues have shown that interference with PPARγ, either systemically or specifically in vascular muscle, produces vascular hypertrophy and inward growth of cerebral arterioles. These findings indicate that PPARγ normally inhibits vascular growth and inward vascular remodelling—effects that have a significant impact on local hemodynamics.
Future research will focus on identifying the mechanisms that promote oxidative stress and alter vascular growth, further defining the cell-specific role for PPARγ, and determining whether overexpression of wild-type PPARγ protects against vascular disease.
Jaroslaw Aronowski, PhD, University of Texas, Houston, TX, reviewed the results of several animal studies that focused on the neuroprotective, cytoprotective, and neuroinflammation role of PPARγ following an intracerebral hemorrhage (ICH).
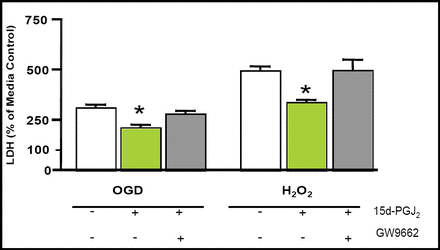
Employing an excitotoxic, ischemia-like (oxygen-glucose-deprivation; OGD), or oxidative stress (hydrogen peroxide; H2O2) injury to neurons, Aronowski and colleagues have shown that PPARγ significantly reduces neuronal death (Figure 1). This neuroprotective effect was linked to increased PPARγ DNA-binding activity [Brain Res. 2006].
PPARγ Activator Reduces ODG- and H2O2-Mediated Damage.
In another study, injection of 15d-Delta(12,14)-prostaglandin J(2) (15d-PGJ(2)), a proposed endogenous PPARγ agonist, into the locus of striatal hematoma increased PPARγ DNA-binding activity, the expression of catalase messenger ribonucleic acid (mRNA), and protein in the perihemorrhagic area. Additionally, 15d-PGJ(2) significantly reduced nuclear factor-kappaB (NFκB) activation and prevented neutrophil infiltration, reduced neuronal death, and reduced behavioral dysfunction produced by the ICH [Zhao X et al. J Cereb Blood Flow Metab 2006].
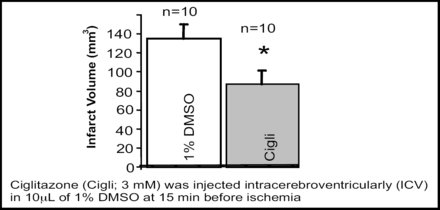
The PPARγ agonist rosiglitazone stimulated primary microglia in culture toward phagocytosis of red blood cells. Rosiglitazone also promoted hematoma resolution, decreased neuronal damage, and improved functional recovery in a mouse ICH model. PPARγ activators significantly increase PPARγ-regulated gene expression (catalase and CD36) and reduce proinflammatory gene expression [Zhao X et al. Ann Neurol 2007]. Intraventricular injection of ciglitazone or 15d-PGJ(2) into ischemic rat brains significantly increased the PPARγ DNA-binding activity and reduced infarction volume at 24h after reperfusion [Figure 2; Ou Z et al. Brain Res 2006].
Intraventricular Injection of Ciglitazone Reduced Infarct Volume 24h After Reperfusion.
All of these results suggest that PPARγ may be beneficial in protecting brain cells from ICH-induced damage. These positive results in animal stroke models have encouraged the experimental study of PPARγ in patients.
Maria A. Moro, PhD, Universidad Complutense, Madrid, Spain, reviewed the results of several animal studies that focused on the neuroprotective and anti-inflammatory role of PPARγ agonists in experimental stroke models and discussed some recent clinical results involving PPARγ ligands that have been shown to improve outcome following ischemic stroke.
Using rats that were exposed to middle cerebral artery occlusion, administration of the PPARγ agonists rosiglitazone, 15d PGJ(2), or L-796,449 after the ischemic onset decreased infarct volume and neuroinflammation, as well as NFκB transcriptional activity [Pereira et al. J Exp Neurol 2005; Pereira et al. J Cereb Blood Flow Metab 2006].
In addition, neuroprotective actions of PPARγ have been shown to go beyond inflammation. A recent study has shown that GLT1/EAAT2, the major glutamate transporter in the central nervous system, is a PPARγ target gene. Upregulation of the expression of this transporter caused by PPARγ activation decreases excitotoxicity and subsequent neuronal death [Romera et al. J Cereb Blood Flow Metab 2007].
In a large group of stroke patients who were admitted within 24 hours of symptom onset, plasma levels of 15dPGJ(2) on admission were significantly higher than in control patients. A linear relationship between increased plasma 15-dPGJ(2) concentration and better neurological outcome at 3 months, less neurological deterioration, and smaller infarct volume was noted, indicating a neuroprotective effect for 15-dPGJ(2) in atherothrombotic ischemic stroke [Blanco M et al. Stroke 2005].
In another clinical study, the use of PPARγ was associated with enhanced functional recovery in stroke patients with type 2 diabetes compared with a control group [Lee J, Reding M. Neurochem Res 2007].
Dr. Moro feels that experimental evidence together with these early clinical results shows a need for larger clinical studies that use PPARγ agonists as potential therapeutic agents not only for prevention but also for treatment of acute stroke.
- © 2008 MD Conference Express