Summary
Does understanding the genotype/phenotype of hypertrophic cardiomyopathy (HCM) influence management of the disease in relation to treatment, risk, and counseling? This article discusses the potential clinical value of molecular diagnosis in HCM. The most important contribution of mutation analysis is clarification of diagnosis rather than preclinical diagnosis (testing of asymptomatic carriers).
- inflammatory disease genomics
Does understanding the genotype/phenotype of hypertrophic cardiomyopathy (HCM) influence management of the disease in relation to treatment, risk, and counseling? William J. McKenna, MD, Heart Hospital, London, UK, addressed this question in the 2007 Rene Laennec Lecture on Clinical Cardiology. Prof. McKenna, whose research has focused primarily on the areas of cardiomyopathy and gene identification in inherited cardiovascular disease, discussed the potential clinical value of molecular diagnosis in HCM. He said that the most important contribution of mutation analysis is clarification of diagnosis rather than preclinical diagnosis (testing of asymptomatic carriers).
Diagnosis of HCM
Distinguishing among the various causes of left ventricular hypertrophy (LVH)—physiologic conditions, metabolic diseases, or genetic syndromes—can be a challenge, said Prof. McKenna. He pointed out that the majority of individuals carrying a disease-causing gene do not have the “classic presentation” of HCM, ie, structural or hemodynamic evidence of LVH on echocardiography. Instead, the diagnosis of familial HCM relies on the microscopic evidence of myocyte disarray, which has been found even in individuals in whom the heart weight and left wall thickness are normal.
Genetic Mutations in HCM
Twelve genes have been identified in HCM. The most important of these are beta-myosin heavy chain and myosin-binding protein C, each of which accounts for 15–30% of cases of familial HCM. Troponin T and troponin I account for 5% and 4% of cases, respectively. The literature suggests that HCM is genetically and phenotypically heterogeneous, said Prof. McKenna, but he believes that HCM caused by different mutations are related disorders. To support his belief, he described cases that illustrated the similarities and differences of the four most common HCM-related genetic mutations.
With beta-myosin heavy chain mutations, there is allelic heterogeneity in relation to morphology, symptoms, and prognosis, with manifestations that range from mild to severe. However, Prof. McKenna noted, “The heterogeneity is between the genes, not within the families.” Disease expression is homogeneous within each family, he explained. Thus, if a parent has mild disease, an offspring will have mild disease. In contrast, disease expression with troponin I mutation is heterogeneous within a family. “You can't predict the risk for the offspring on the basis of the parent. If the parent has mild disease, you can't assume that the child will have mild disease as well,” he said.
With troponin T mutations, symptoms are mild or even absent. However, the prognosis is poor, with a risk of premature sudden death. He added that postmortem analyses of cardiac tissue have shown 2 to 3 times the amount of myocyte disarray, indicating that this finding may be a marker of electrical instability in patients with troponin T mutation.
Myosin-binding protein C mutations are also homogeneous within families, but unlike the other disease-causing genes, this gene is associated with late-onset disease expression. Prof. McKenna said that the disease associated with this mutation has been referred to as mild; however, he pointed out, “It's mild only in that it occurs late in life. Once it manifests, the patient has the same disease-related complications, including arrhythmias, emboli, and sudden death.”
Influence of Mutation Analysis on Management
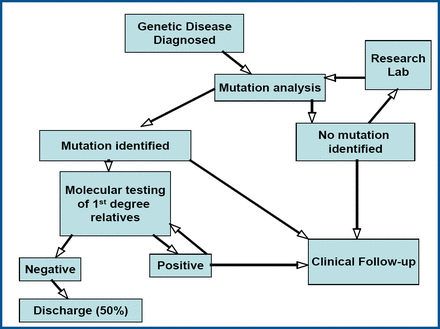
How is mutation analysis applied in clinical practice? Prof. McKenna described a pathway for testing that involves carrying out mutation analysis on first-degree relatives when a mutation is detected in an individual (Figure 1). “If a mutation is found, you know that serial follow-up has merit,” he said. If no mutation is found, the patient is discharged. Genetic counseling should be offered when testing demonstrates troponin I or beta-myosin heavy chain mutations. Perhaps the most important illustration of how knowledge of a specific mutation influences management is the identification of an individual with a troponin T mutation and being able to avoid premature sudden death with an implantable defibrillator.
Molecular Diagnostics in Clinic.
Advantages and Disadvantages of Mutation Analysis
Determining a molecular diagnosis of familial HCM has many advantages as well as some disadvantages, said Prof. McKenna. The targeted management allowed by a molecular diagnosis is cost-effective, as serial follow-up is done only when warranted by risk. Diagnostic uncertainty and false-negative clinical evaluations are also avoided, leading to better psychological well-being for patients. The primary disadvantage is related to the complexity of testing. For example, genetic data are difficult to interpret, an individual may have more than one disease-causing gene, and evaluation of entire families is needed. In addition, the approach requires that cardiologists work with genetic counselors and other specialists. “We will need new models of care,” he said.
- © 2007 MD Conference Express