Summary
Ranolazine, an anti-ischemic agent approved for use in the treatment of chronic angina, has also been shown to have anti-arrhythmic effects in experimental models. The MERLIN-TIMI 36 trial was the first study in which the anti-arrhythmic effects of ranolazine were evaluated in humans.
- cardiology clinical trials
- arrhythmias
Ranolazine, an anti-ischemic agent approved for use in the treatment of chronic angina, has also been shown to have anti-arrhythmic effects in experimental models. The MERLIN-TIMI 36 trial was the first study in which the anti-arrhythmic effects of ranolazine were evaluated in humans.
MERLIN-TIMI 36 involved 6,560 patients with unstable angina/non-ST-elevation acute coronary syndrome who were randomly assigned to receive ranolazine or placebo in addition to standard therapy. The primary results of the study were recently published [Morrow DA et al. JAMA 2007]. In a major trial substudy, continuous 3-lead electrocardiography recordings were obtained for 6,351 patients during the first 7 days after randomization.
Benjamin Scirica, MD, Brigham and Women's Hospital, Boston, Massachusetts, reported the findings of the arrhythmia endpoints analysis within MERLIN-TIMI 36. Dr. Scirica reviewed the novel mechanism of action of ranolazine, an inhibition of the late phase of the sodium current, one consequence of which is a reduction of the detrimental electrophysiologic effects associated with intracellular sodium and calcium overload in the myocardium [Antzelevitch C et al. Circulation 2004]. Because ranolazine is known to cause a slight prolongation of the QT interval, there was concern that ranolazine might have pro-arrhythmic effects such as had been observed with other drugs that prolong the QT interval such as some class IC anti-arrhythmic, fluoroquinolone antibiotics, and anti-histamines.
Dr. Scirica reported that evaluation of the electrocardiography recordings for a prespecified set of arrhythmias demonstrated that ranolazine resulted in significantly fewer episodes of ventricular tachycardia, supraventricular tachycardia, and bradyarrhythmias (Table 1).
Comparison of Arrhythmia Endpoints Associated with Ranolazine and Placebo in MERLIN-TIMI 36.
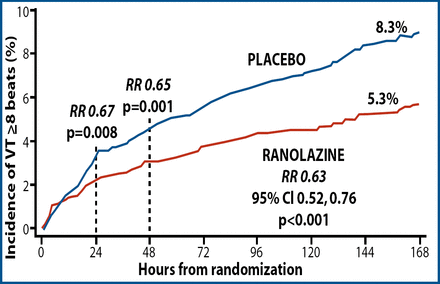
Ranolazine led to a 37% decrease in the risk of ventricular tachycardia of 8 beats or more (Figure 1). This significant effect occurred early, within 24 hours after treatment with ranolazine began, and persisted throughout the 7 days of monitoring. He added that the significant reduction in ventricular tachycardia persisted when the defined duration was extended to 10, 15, and 20 or more beats.
First Occurrence of Ventricular Tachycardia Lasting ≥ 8 Beats.
Copyright © 2007. American Heart Association. All rights reserved.
The positive effect of ranolazine was consistent across several high-risk subgroups based on ejection fraction, corrected QT interval, TIMI risk score, history of heart failure, and the presence or absence of ischemia on electrocardiography. In addition, there was no evidence of a significant excess risk in either life-threatening arrhythmias, such as polymorphic ventricular tachycardia, or sudden cardiac death in patients treated with ranolazine.
The most “impressive and noteworthy” finding according to A. John Camm, MD, St. George's Hospital Medical School, London, UK, who discussed the study, was the significant decrease in the incidence of ventricular tachycardia of 8 beats or more among patients with an ejection fraction of less than 40 (16.6% vs 8.8%; p=0.001). Dr. Camm further commented, “This trial suggests that ranolazine is anti-arrhythmic rather than pro-arrhythmic. It is very impressive and seriously adds to the convincing safety database ranolazine.”
The full study report was published online on 5 September 2007 (Circulation. 2007;116:000–000) and is available at and is available at: http://circ.ahajournals.org/
- © 2007 MD Conference Express