Summary
Results of 3 years of clinical follow-up data continued to show superiority for a combination of tirofiban+sirolimus-eluting stent (SES) vs a combination of abciximab+bare-metal stent (BMS) in STEMI patients, as evidenced by significantly reduced target vessel revascularization (TVR) rates.
- cardiology clinical trials
- myocardial infarction
- interventional techniques & devices
Results of 3 years of clinical follow-up data presented by Marco Valgimigli, MD, University of Ferrara, Italy, continued to show superiority for a combination of tirofiban+sirolimus-eluting stent (SES) vs a combination of abciximab+bare-metal stent (BMS) in STEMI patients, as evidenced by significantly reduced target vessel revascularization (TVR) rates.
In the STRATEGY (Single High Dose Bolus Tirofiban and Sirolimus-Eluting Stent vs Abciximab and Bare Metal Stent in Myocardial Infarction) trial, 175 patients with STEMI were randomly assigned to receive a single high-dose bolus of tirofiban (25 μg/kg/3 min) and infusion (0.15 μg/kg/min for 18–24 hours) followed by SES implantation (n=87), or abciximab (bolus of 0.25 mg/kg/3-min with 0.125 mg/kg/min for12 hours) followed by BMS implantation (n=88).
The initial results through 8 months showed that treatment with tirofiban+SES was associated with a reduction in the primary endpoint (death, myocardial infarction (MI), stroke, or binary restenosis) vs the use of abciximab+BMS [Valgimigli M et al. JAMA 2005]. The 2-year study results published earlier this year [Valgimigli M et al. J Am Coll Cardiol 2007] showed a continued superiority of the tirofiban+ SES combination.
3-Year Results
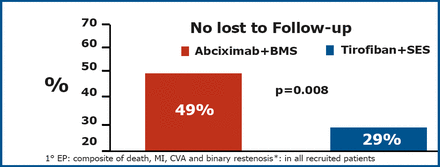
A total of 74 (85%) patients in the tirofiban+SES and 77 (88%) in the abciximab+BMS arm were still being followed at 3 years. The primary endpoint was significantly lower in the tirofiban+SES group (p=0.008) (Figure 1).
Primary Endpoint at 3 Years.
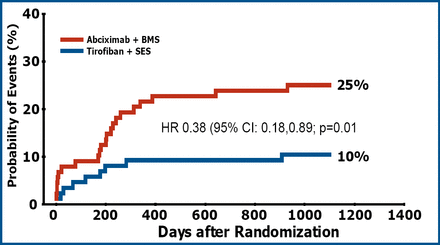
This difference was driven by an improvement in angiographic outcome leading to a significant reduction in the need for TVR in the tirofiban+SES group (p=0.01) (Figure 2).
TVR at 3 Years.
The cumulative incidence of major adverse cardiac events (death, MI or TVR) was lower in the tirofiban+SES group (28.7%) vs abciximab+BMS (40.9%) although the difference was not significant. All-cause mortality (16.0% vs 14.7%, p=0.85 and the composite of death/MI (19.5% vs 22.7%, p=0.57) were similar between the two treatment groups (tirofiban+SES and abciximab+BMS, respectively). There was no difference in the cumulative incidence of stent thrombosis (5.7% in the tirofiban+SES and 6.8% in the abciximab+BMS group, p=0.76).
“The results are reassuring given that the original results have not changed much over time,” said Dr. Valgimigli. “Yet, we should not forget the number of patients in this study was very low.” Further large scale trials are necessary to establish the role of this proposed strategy and to evaluate whether the observed differences in this study were related to the type of stent utilized or the selected GP IIb/IIIa antagonist. Future studies should also include data on the duration of dual antiplatelet treatment and the immediate consequences of its termination
- © 2007 MD Conference Express