Summary
Ranolazine is an anti-ischemic agent indicated for the treatment of chronic angina. Its effects occur without clinically significant changes in heart rate or blood pressure. However, because ranolazine is associated with a mild prolongation of the QTc interval (mean change approximately 6 ms), it currently is indicated only for patients who have not responded to other therapies. Because of this potentially worrisome prolongation of the QT interval, additional safety data were sought.
- Cardiology Clinical Trials
- Myocardial Infarction
Ranolazine is an anti-ischemic agent indicated for the treatment of chronic angina. Its effects occur without clinically significant changes in heart rate or blood pressure. However, because ranolazine is associated with a mild prolongation of the QTc interval (mean change approximately 6 ms), it currently is indicated only for patients who have not responded to other therapies. Because of this potentially worrisome prolongation of the QT interval, additional safety data were sought. David Morrow, MD, MPH, of Brigham and Women's Hospital, gave an overview of the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndrome (MERLIN TIMI 36) study. The study had three main objectives: 1) to assess acute efficacy of ranolazine in acute coronary syndrome (ACS) by determining the potential for a decrease in major cardiovascular events, 2) to assess chronic efficacy of the drug for secondary prevention and relief of angina and 3) to evaluate the safety of the compound in the acute and chronic setting.
The trial included patients hospitalized with non-ST-elevation ACS with ischemic symptoms at rest and at least one of four features indicating moderate to high risk: 1) an increase in troponin (myocardial infarction limit) or creatinine kinase -MB (upper limit of normal); 2) ST-depression ≥0.1mV; 3) diabetes mellitus, or; 4) a TIMI risk score for unstable angina/non-ST-elevation myocardial infarction ≥3.
A total of 6,550 patients were randomly assigned in a 1:1 ratio to one of two treatment groups: 1) ranolazine IV 200 mg over one hour, followed by 80 mg/hour infusion for up to 96 hours, followed by ranolazine 1000 mg/day PO or; 2) IV and oral placebo given in an identical fashion. Patients were monitored by continuous Holter for one week. The primary endpoint was a composite of cardiovascular death, new/recurrent myocardial infarction (MI), and recurrent ischemia. The primary endpoints were adjudicated by a blinded cardiovascular events committee.
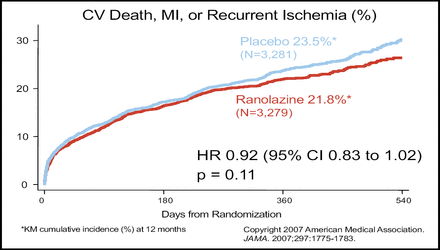
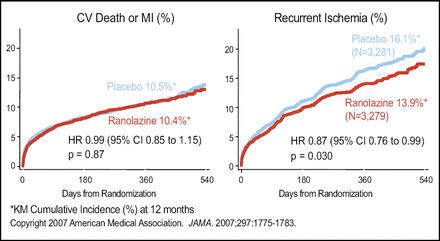
The baseline demographic characteristics were well balanced between the ranolazine (n=3,279) and the placebo group (n=3,281). Primary endpoint analyses indicated no statistically significant difference in the composite of cardiovascular death, MI, or recurrent ischemia between the two groups (p=0.11; Figure 1). In an analysis of the components of the primary endpoint, ranolazine had no effect on cardiovascular death or MI. However, ranolazine was significantly better than placebo in reducing recurrent ischemia (p=0.03; Figure 2).
Primary Endpoint.
Components of Primary Endpoint.
The safety findings indicated no significant differences in death from any cause, sudden cardiac death, death or cardiovascular hospitalization, or symptomatic documented arrhythmia. Significantly more placebo-treated patients experienced a pre-specified set of arrhythmias on Holter (83.1% vs 73.7%, respectively; p<0.001). Dr. Morrow concluded by saying that ranolazine does not add to standard therapy for acute management of ACS. Ranolazine did not reduce cardiovascular death or MI, but was effective as an anti-anginal, with overall safety findings that were reassuring, including potential anti-arrhythmic effects that deserve further study.
- © 2007 MD Conference Express