Summary
An A2 subtype form of botulinum toxin, A2NTX, is an effective substitute for onabotulinumtoxinA and demonstrates significantly larger decreases in Modified Ashworth Scale ankle plantar extensor at 30 days after injection. Functional independence also significantly improves at 60 days, and there is less toxin spread into other areas of the body following A2NTX injection.
- post-stroke
- lower-limb spasticity
- botulinum toxin
- subtype 1 and 2
- A2NTX
- onabotulinumtoxinA
- Modified Ashworth Scale
- NCT01910363
- neurology clinical trials
- ischemia
In a poster presentation, Ryuji Kaji, MD, University of Tokushima, Tokushima, Japan, reported that A2NTX, a new botulinum toxin preparation derived from botulinum toxin serotype A (BoNT/A), is more effective for treating poststroke lower limb spasticity compared with the A1 subtype of BoNT (onabotulinumtoxinA).
In this phase 2/3 proof-of-concept study [NCT01910363], patients were not prescribed any rehabilitation posttreatment, to reduce the influence of varying rehabilitation intensiveness. Men and women, aged 40 to 79 years, with poststroke lower limb spasticity of > 6 months’ duration were included. Participants had a Modified Ashworth Scale (MAS) score for either flexion or extension ankle joint ≥ 2 and both flexion and extension > 0.
Guided by electromyography, either onabotulinumtoxinA or A2NTX was first injected into the tibialis posterior (150 Units), then into the medial gastrocnemius (150 Units) using the same needle tract on the affected side. Ninety days later, this procedure was repeated. MAS was measured at baseline, 30 (27-33) days, and 60 (56-63) days after injection. Areas under the curve of MAS changes at day 30 and day 60 after injection were compared between treatments. Secondary outcome measures included changes in functional independence measure (FIM) and changes in handgrip measured in kg units. As injections were made in the lower limbs, any decrease of grasp power was assessed as a measure of unwanted spread of the toxin action, and thus a measure of safety and toxin spread.
The men-to-women ratio was higher in the onabotulinumtoxinA group (15:1) compared with the A2NTX group (10:5). Mean age was 65.6 years; duration of illness was 93 months; side of paresis favored the left in the onabotulinumtoxinA group and the right in the A2NTX group; MAS for ankle plantar extension was 2.53 and flexion 0.97; hand grip power was 30 kg; and mean FIM was 26.1 (on a scale of 18-126, with higher scores indicating more independence).
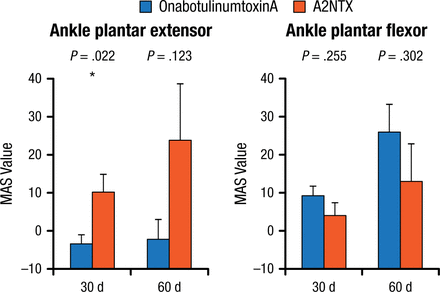
At 30 days, A2NTX significantly improved ankle plantar extensor compared with onabotulinumtoxinA (P = .022), although the same was not true at 60 days. There were no significant differences in flexor values at either 30 or 60 days (Figure 1).
Changes in Extensor and Flexor MAS Values at 30 and 60 Days
Mean ± standard error of AUC, Student t.
AUC, area under the curve; MAS, Modified Ashworth Scale.
*Paired-t analysis.
Reproduced with permission from R Kaji, MD.
FIM was significantly improved at 60 days for patients treated with A2NTX (P = .006) but not onabotulinumtoxinA. Patients in the onabotulinumtoxinA-treated group had a significant reduction of handgrip at 60 days (P = .002), whereas patients in the A2NTX group did not, likely indicating that A2NTX had less distant spread than onabotulinumtoxinA.
The investigators concluded that A2NTX is an effective substitute for onabotulinumtoxinA, with less distant spread.
- © 2015 SAGE Publications