Summary
While antibiotic resistance is a growing problem across the globe, it is not clear whether the predicted trajectory of resistance has been adequately evaluated. Blind adherence to strict practice guidelines regarding antibiotics may put patients at harm, and researchers are urged to appraise the evidence underlying clinical recommendations.
- antibiotic resistance
- community-acquired pneumonia
- clinical guidelines
- antibiotics
- epidemiology
- bacterial infections
- infectious diseases guidelines
Mark J. M. Bonten, MD, University Medical Center Utrecht, Utrecht, The Netherlands, was presented with the 2015 European Society of Clinical Microbiology and Infectious Diseases Award for Excellence in Clinical Microbiology and Infectious Diseases. Prof Bonten is widely recognized for his work in microbial resistance and the prevention of hospital-acquired infections, and he offered a thought-provoking perspective regarding the cause, effects, and possible solutions to antimicrobial resistance (AMR).
Prof Bonten began by reviewing the history of antibiotic use in Dutch intensive care units (ICUs). Since the 1980s, hospitals have practiced selective digestive decontamination (SDD), designed to prevent transmission of gram-negative bacteria, Staphylococcus aureus, and yeasts associated with ICU-acquired infections and to prophylactically treat community-acquired respiratory tract infections.
The antibiotics used for SDD include an oropharyngeal paste of polymyxin E, amphotericin B, and tobramycin 4 times per day. These same antibiotics are also given via an intragastric tube. Patients also receive 1 g of intravenous cefotaxime for 4 days. The use of antibiotics that kill anaerobes are discouraged during this regimen. A variation of SDD (selective oropharyngeal decontamination [SOD]) is used in some parts of The Netherlands and includes only the oropharyngeal paste portion of the regimen, with no restriction on the physicians’ choice of antibiotics.
Prof Bonten then reviewed data from studies that investigated clinical outcomes of SDD and SOD. Two studies comprising almost 18 000 patients compared SDD and SOD to standard care [Oostdijk EA et al. JAMA. 2014; de Smet AM et al. N Engl J Med. 2009]. While fewer deaths occurred at day 28 in both the SDD and SOD groups compared with standard care, neither SOD nor SDD was associated with a significant difference in any other clinical outcome. In an analysis comparing the de Smet trial with 2 smaller studies [De Jonge E. et al. Lancet. 2003; Bergmans DC et al. Am J Respir Crit Care Med. 2001], SOD/SDD provided no benefit on ICU mortality, 28-day mortality, and hospital mortality with standard care. Of note, however, is that cephalosporin use increased by > 85% in patients who underwent SDD, suggesting that the cefotaxime used in the SDD protocol might be responsible for an antibiotic resistance [de Smet AM et al. N Engl J Med. 2009].
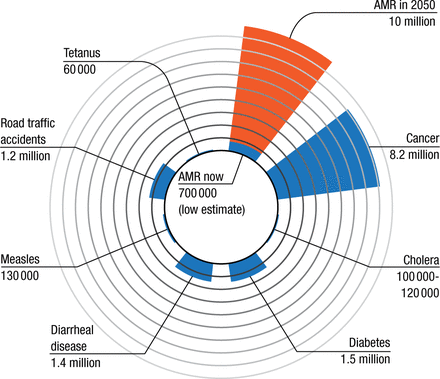
According to Prof Bonten, approximately 25 000 patients die in the European Union each year from infection due to resistant pathogens [Norrby R et al. The Bacterial Challenge: Time to React. European Centre for Disease Prevention and Control and European Medicines Agency, 2009]. In the United States, the CDC estimates that > 2 million people will experience an antibiotic-resistant infection each year and that at least 23 000 will die as a result [US Department of Health and Human Services. Antibiotic Resistance Threats in the United States. Washington, DC, 2013]. Some data suggest that while there are currently 700 000 global deaths attributable to AMR, this number will rise to > 10 million by 2050 (Figure 1) [Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014]. Most of the mortality is predicted to occur in Asia and Africa.
Deaths Attributable to AMR Every Year
AMR, antimicrobial resistance.
Sources: Lozano R et al. The Lancet. 2012; WHO Media Centre, 2014-2015.
Adapted from ‘Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014’ by JPIAMR, licensed under CC BY 4.0, which is available at https://creativecommons.org/licenses/by/4.0/
These predictions are based on the results of epidemiologic modeling. However, Prof Bonten explained that while epidemiologic studies try to make an association between antibiotic resistance and outcome, they often do not take into account the etiology of the health outcome of interest. In the case of antibiotic resistance, there are many factors—including exposure to both health care and antibiotics, as well as underlying disease—that have an impact on patient outcomes.
Prof Bonten then reviewed data from several studies suggesting that multiple factors are responsible for poor outcomes in patients with methicillin-resistant S aureus (MRSA). In 1 trial, inadequate empirical antimicrobial therapy was responsible for an increased incidence of MRSA but was not associated with an increased 30-day mortality [Ammerlaan H et al. Clin Infec Dis. 2009]. Another study reported that patients with MRSA had poorer long-term outcomes than patients with methicillin-sensitive S aureus, due to confounding associations with factors such as age, comorbidities, metastatic infections, and severity of acute illness [Yaw LK et al. Lancet Infec Dis. 2014]. After adjusting for these factors, there was no significant difference in infection-related hospital admissions, all-cause mortality, or infection-related mortality between the 2 groups.
Prof Bonten closed this section of his presentation by emphasizing that epidemiologists have not yet accurately quantified the consequences and causes of antibiotic resistance as they relate to patient outcomes. Therefore, it is not really possible to know whether the projections of effects directly attributable to antibiotic resistance are overblown or whether the increase in the total burden of infections is higher than currently estimated.
Prof Bonten then moved on to a discussion about clinical guidelines for treating community-acquired pneumonia (CAP). In general, physicians cannot predict the causative pathogen at the time that antibiotics are prescribed, so they use disease severity classifications such as the Pneumonia Severity Index (PSI) or the CURB-65 to guide treatment. The Dutch treatment guidelines for patients with CAP are shown in Table 1.
Disease Severity and Empirical Treatment for Community-Acquired Pneumonia
There is a paucity of data, however, to confirm whether there is any agreement among the classifications in determining disease severity and whether the classification system results in undertreatment. Prof Bonten highlighted a Dutch observational study suggesting that concordance among the 3 CAP severity classifications (PSI, CURB-65, and pragmatic) was low (26%); patients were most likely to receive the appropriate treatment based on the pragmatic score (63%) as opposed to the CURB-65 score (30.5%) [Huijts SM et al. Neth J Med. 2013]. Furthermore, nonadherence to treatment recommendations based on PSI and CURB-65 did not result in poor outcomes. In another Dutch study, patients with clinically suspected CAP admitted to non-ICU wards showed similar clinical outcomes regardless of whether they received β-lactam monotherapy, a β-lactam/macrolide combination, or fluoroquinolone monotherapy [Postma DE et al. N Engl J Med. 2015]. Prof Bonten concluded that while guidelines based on scientific evidence have improved the quality of medical care, such guidelines may be harmful if there is not enough evidence to validate their utility. He also urged colleagues from other parts of the world to scrutinize their countries’ guidelines, as the Dutch experience is likely not that different from that of other countries.
In closing, Prof Bonten proposed a number of solutions to the problem of AMR. He focused on public-private partnerships and cited as an example the New Drugs for Bad Bugs initiative, which was launched by the European Commission in 2011 with a mission of delivering a pipeline of new antibacterial agents to patients. The partnership includes representatives of pharmaceutical companies, academia, and representatives from various European governments, and it eventually hopes to address some of the challenges associated with antibacterial drug discovery, development, and commercialization that are common across Europe. He also reminded attendees that antibiotics cause more than resistance, that we need more research to quantify the scope of antibiotic resistance throughout the world, and that adhering to strict guidelines without questioning their validity may not always be in the best interest of patients.
- © 2015 SAGE Publications