Summary
In the ADVANZ-3 trial, among patients with advanced HIV-1, 96 weeks of daily combination treatment with efavirenz plus tenofovir/emtricitabine is as effective in increasing CD4+ T-cell levels as daily combination treatment using atazanavir/ritonavir plus tenofovir/emtricitabine or lopinavir/ritonavir plus tenofovir/emtricitabine. Similar improvements are also seen for inflammation, coagulation, and bacterial translocation markers.
- efavirenz
- HIV-1
- ADVANZ-3
- NCT00532168
- atazanavir/ritonavir
- lopinavir/ritonavir
- tenofovir/emtricitabine
- combined antiretroviral therapy
- HIV & AIDS
- infectious diseases clinical trials
Final results of the ADVANZ-3 trial [NCT00532168], presented by Christian Manzardo, MD, PhD, Hospital Clinic-IDIBAPS, Barcelona, Spain, confirmed that efavirenz (EFV)-based therapy is appropriate for HIV-1–infected patients with very low CD4 T-cell counts and high plasma HIV-1 RNA levels, provided they are adherent to therapy and do not have transmitted drug-resistant mutations.
It is not known whether the type of combination antiretroviral therapy (cART) regimen (ie, a protease inhibitor [PI]–based vs nonnucleoside reverse transcriptase inhibitor–based regimen) impacts treatment outcomes in highly suppressed patients with HIV-1. In addition, only limited data exist concerning the effects of cART on bacterial translocation, inflammation, coagulation, and immune activation in patients with advanced HIV-1 infection.
The ADVANZ-3 trial was a randomized, controlled, open-label, multicenter phase 4 study to compare the immunological reconstitution and the virologic efficacy and safety of 3 different combinations of antiretroviral therapy given once a day. The study included antiretroviral-naïve, HIV-1–infected adults with very low CD4 cell counts (< 100 CD4 cells/mm3) and no drug resistance mutations at baseline.
Participants were randomized 1:1:1 to EFV 600 mg QD (n = 29), atazanavir/ritonavir 300/100 mg QD (n = 30), or lopinavir/ritonavir (LPV/r) 400/100 mg BID (n = 30) in addition to tenofovir/emtricitabine QD for 96 weeks. The primary study outcome was the median increase in the CD4+ T-cell count at week 48. Secondary end points included the proportion of patients with plasma HIV-1 viral load < 50 copies/mL; the incidence of side effects; disease progression and death; and changes in the markers of immune activation and senescence, apoptosis, inflammation, bacterial translocation, and coagulation.
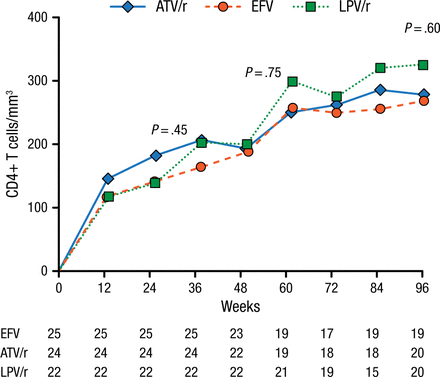
At baseline, participants (mean age 38 years; 82% men) had a median CD4 cell count of 34 cells/mm3 and a median plasma viral load of 5.26 log10/mL. After 96 weeks, all 3 treatments were associated with increases in CD4+ T-cell counts (+284 cells/mm3 in the EFV arm, +295 in the atazanavir/ritonavir group, and +345 among those treated with LPV/r; Figure 1).
Median Increase in CD4+ T-Cell Counta
ATV/r, atazanavir/ritonavir; EFV, efavirenz; LPV/r, lopinavir/ritonavir.
aOn-treatment analysis.
Reproduced with permission from C Manzardo, MD, PhD.
The percentages of patients achieving viral suppression on both the intention-to-treat and on-treatment analyses were similar (intention-to-treat 75%, 60%, and 58.6% and on-treatment 100%, 100%, and 90% for EFV, atazanavir/ritonavir, and LPV/r, respectively), as were decreases in the levels of inflammation, coagulation, and bacterial translocation markers. The incidence rate of adverse events was similar in the 3 groups; there were no deaths.
Additional studies are needed in this patient population for other first-line regimens such as those using other boosted PIs (eg, darunavir) or integrase inhibitors (eg, dolutegravir).
- © 2015 SAGE Publications