Summary
Catheter ablation increasingly is being used to manage patients with atrial fibrillation. Symptomatic patients should be considered for ablation soon after antiarrhythmics have been unsuccessful. Patients should continue anticoagulation for 3 months following the procedure and should undergo postprocedure follow-up as suggested by published guidelines.
- atrial fibrillation
- catheter ablation
- pulmonary vein isolation
- heart failure
- amiodarone
- antiarrhythmics
Catheter ablation has been used to treat heart rhythm disorders for > 20 years and is increasingly used in the management of patients with atrial fibrillation (AF). Guidelines published by the European Society of Cardiology (ESC) currently recommend catheter ablation as an alternative to antiarrhythmic drug therapy for patients with symptomatic recurrent paroxysmal AF with no or minimal structural heart disease, provided the procedure is performed by an experienced operator [Camm J et al. Eur Heart J. 2012]. More commonly, it is used as an accepted rhythm control therapy in patients in whom antiarrhythmic drugs are not effective.
Dipen Shah, MD, University Hospitals of Geneva, Geneva, Switzerland, chaired a session designed to help physicians manage patients with AF who undergo catheter ablation, who have previously undergone such a procedure, or who are considering such a procedure. The session was designed to review controversial issues surrounding the use of catheter ablation as a first-line therapy of rhythm control—which patients are the best candidates for the procedure, optimal strategies to prepare the patient for the procedure, whether to stop antiarrhythmic drugs or anticoagulants, and what to do when faced with the possibility of recurrence. Prof Shah also emphasized the importance of long-term follow-up for these patients, some of whom may have a fluctuating or progressive course.
Prof Shah then introduced 3 speakers who presented case reports highlighting key aspects of clinical importance, while a fourth speaker provided a take-home message for attendees.
Jens Cosedis Nielsen, MD, PhD, Aarhus, Denmark, described the case of a 56-year-old male patient with highly symptomatic paroxysmal AF who experienced weekly episodes of 1 to 6 hours’ duration that left him unable to work. His electrocardiogram was normal with sinus rhythm, but his echocardiogram revealed marginal left ventricular hypertrophy. Metoprolol was not well tolerated and was ineffective in reducing the burden of arrhythmias. A Holter monitor showed multiple episodes of AF at 140 to 150 beats per minute and short bursts of atrial tachycardia.
Prof Nielsen led the audience through the evidence to determine whether this patient would be a candidate for catheter ablation as first-line treatment for paroxysmal AF. Citing data from the MANTRA-PAF trial [Cosedis Nielsen J et al. N Engl J Med. 2012], he emphasized that there were no significant differences in the cumulative burden of AF over 2 years among 294 patients who underwent catheter ablation or who received antiarrhythmic drug therapy (P = .10). He also reviewed data from the RAAFT2 study [Morillo CA et al. JAMA. 2014], which showed significantly fewer recurrences of any atrial tachyarrhythmias in patients who underwent catheter ablation compared with those who received antiarrhythmic drugs at 2 years (HR, 0.60; 95% CI, 0.35 to 0.90); of note is that recurrence was frequent in both groups.
Prof Nielsen emphasized the homogeneity of the patients in both trials—mostly men with a mean age of 55 years, a normal left ventricular ejection fraction (LVEF), and a left atrial diameter of approximately 4 cm. Less than half were hypertensive and the average CHADS2 score was < 2. Very few of the patients had diabetes, a prior stroke, or coronary artery disease. According to Prof Nielsen, candidates similar to this relatively healthy group of patients would be good candidates for catheter ablation.
He also reminded attendees to thoroughly review the complications of both treatments with patients who might be appropriate candidates for the procedure (Table 1).
Complications of Catheter Ablation and Antiarrhythmic Drugs
After receiving information about the benefits and risks of both strategies, the patient chose to undergo catheter ablation with isolation of the pulmonary vein. At 12 months, Holter monitoring detected no AF and there was no recurrence of arrhythmia at 18 months. Prof Nielsen closed his presentation with a reminder that catheter ablation is not a cure for most patients with AF and that it is not a risk-free procedure.
Paulus Kirchhof, MD, University of Birmingham Institute of Cardiovascular Sciences, Birmingham, UK, presented the case of a 66-year-old man with recent-onset persistent symptomatic AF and atrial flutter who underwent catheter ablation after failed rhythm control with electrical cardioversion. The patient was switched to warfarin prior to the ablation, per the 2012 update of the ESC AF guidelines [Di Biase L et al. Circulation. 2014; Camm AJ et al. Europace. 2012].
Although the patient did require an additional ablation for atrial flutter, the therapy otherwise resulted in good symptom control. Catheter ablation for persistent AF is less effective than for paroxysmal AF but can be considered for symptomatic persistent AF resistant to antiarrhythmic drugs (Class IIa recommendation) or patients with long-standing persistent AF.
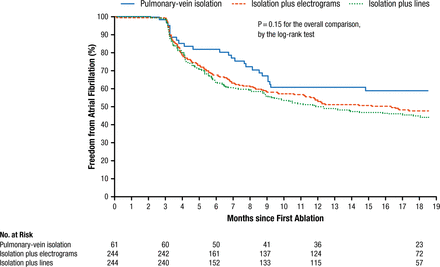
Prof Kirchhof noted that catheter ablation is less often successful for persistent AF than for paroxysmal AF. Although some guidelines have suggested that pulmonary vein isolation (PVI) be augmented with electrograms or lines, recently published data suggest that PVI may be initially sufficient for persistent AF (Figure 1) [Verma A et al. N Engl J Med. 2015], possibly reducing the risk of left atrial flutters in the future.
Freedom From Atrial Fibrillation
From N Engl J Med, Verma A et al, Approaches to catheter ablation for persistent atrial fibrillation, Vol 372, Pages 1812-1822, Copyright © (2015) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
In a presentation highlighting the presence of AF in patients with heart failure, Philippe Mabo, MD, University Hospital, Rennes, France, described the case of a patient with persistent AF and an underlying dilated cardiomyopathy with symptoms of heart failure and a low ejection fraction. Although AF and heart failure frequently coexist, the prevalence of AF increases with severity of heart failure, ranging for 5% in NYHA Class I to 50% in NYHA Class IV patients. According to Prof Mabo, patients with heart failure are more likely to have a persistent (or permanent) AF.
Prof Mabo described the case of a 55-year-old man with a 2-year history of a dilated nonischemic cardiomyopathy, on optimal medical treatment. As his symptoms worsened, the patient underwent a catheter ablation procedure after failed rhythm control with amiodarone and electrical cardioversion. According to Prof Mabo, the decision to move ahead with the catheter ablation was based on the 2012 ESC indications for AF ablation in patients with structural heart disease, which recommend that catheter ablation is a first-line option alongside amiodarone for patients whose heart failure is due to AF and as a second-line option after amiodarone in patients whose heart failure cannot be attributed to AF [Camm J et al. Eur Heart J. 2012].
Prof Mabo then described an ongoing clinical trial that compares catheter ablation with amiodarone for the treatment of AF in patients with congestive heart failure and an implanted cardiac resynchronization therapy device [NCT00729911].
Returning to his original case, Prof Mabo reported that after an extensive ablation procedure, the patient required cardioversion to restore normal sinus rhythm. Two months later, he underwent a repeat ablation. Six months later, he was NYHA Class I with stable sinus rhythm and an LVEF of 35%. Amiodarone was stopped and oral coagulation was maintained. At 1 year after the redo procedure, he had no recurrence of AF, a stable LVEF of 35%, and NYHA Class I symptoms.
Prof Mabo concluded his presentation by emphasizing that ablation of persistent AF of short duration may be considered as a first-line therapy in patients with left ventricular dysfunction when a functional and/or symptomatic improvement is expected. The procedure should have no impact on long-term anticoagulation and repeat ablations are frequently required. It is still not clear whether the best strategy should be PVI or more complex procedures or what the long-term effect of ablation on LV function, or the impact of catheter ablation on mortality might be.
Christian de Chillou, MD, University Hospital, Nancy, France, summarized the session by emphasizing that clinicians carefully think about which patients should be referred for catheter ablation and when they should be referred. He suggested that ablation of AF early in the course of the disease might halt progression to persistent AF. He underlined the fact that early complications can occur after catheter ablation and that oral anticoagulation before, during, and after catheter ablation is very important. He urged continued follow-up of these patients in order to ensure optimum control of risk factors and protection from thromboembolic events.
Prof de Chillou then reviewed recommendations for minimum follow-up screening of patients who undergo catheter ablation. For paroxysmal AF, the minimum follow-up screening should include (1) 12-lead ECG at each follow-up visit; (2) 24-hour Holter at the end of the follow-up period (eg, 12 months); and (3) event recording regularly and at the time of symptoms with an event monitor from the end of the 3-month blanking period to the end of follow-up (eg, 12 months). For persistent or longstanding AF recurrence, the minimum follow-up screening should include (1) 12-lead electrocardiogram at each follow-up visit; (2) 24-hour Holter every 6 months; and (3) symptom-driven event monitoring [Calkins H et al. Europace. 2012].
He summarized his presentation with 3 points:
Symptomatic patients should be referred for AF ablation early on, after antiarrhythmic medication has been unsuccessful.
Anticoagulation should be continued for 3 months postablation and should not be interrupted in patients with a CHA2DS2-VASC score ≥ 2.
Patients should undergo follow-up screening per published guidelines for complications and AF recurrences.
- © 2015 SAGE Publications