Summary
There are now 4 novel oral anticoagulants available as an alternative to vitamin K antagonists for the risk reduction of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. However, many unanswered questions remain about their use in clinical practice, and numerous trials are ongoing.
- ARISTOTLE
- ENGAGE AF-TIMI 48
- RE-LY
- ROCKET AF
- anticoagulation
- arrhythmias
- atrial fibrillation
- cardiology guidelines
- NOACs
- oral anticoagulation
- stroke
- warfarin
The prevalence and mortality related to atrial fibrillation (AF) have increased over the past 3 decades [Chugh SS et al. Circulation. 2014]. Importantly, AF is associated with an increased risk of stroke, but anticoagulation has been shown to be an effective method of decreasing the risk of stroke [Hart RG et al. Ann Intern Med. 2007]. Since 2009, 4 novel oral anticoagulants (NOACs) that offer an alternative to vitamin K antagonists such as warfarin have been approved by the FDA for the prevention of stroke in patients with nonvalvular AF.
In a recent meta-analysis, NOACs were shown to decrease the risk of stroke or stroke and systemic embolism (SSE) as compared with warfarin, noted Christian T. Ruff, MD, MPH, Brigham and Women’s Hospital, Boston, Massachusetts, USA [Ruff CT et al. Lancet. 2014]. In addition, the NOACs decreased the risk of major bleeding when compared with warfarin. Specifically, treatment with a NOAC resulted in markedly fewer intracranial hemorrhages but more gastrointestinal bleeding as compared with warfarin.
As a result of the data demonstrating the efficacy and safety of the NOACs, the current clinical practice guidelines for the management of AF published by the European Society of Cardiology and the American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) recommend that all patients with nonvalvular AF with a CHA2DS2-VASc score ≥ 2 should receive anticoagulation, with a NOAC preferred over warfarin [McMurray JJV et al. Eur Heart J. 2012; January CT et al. J Am Coll Cardiol. 2014]. Patients with a mechanical heart valve should receive warfarin. Both guidelines agree that patients with a CHA2DS2-VASc score of 0 should not receive anticoagulation; however, the European Society of Cardiology guidelines recommend that patients with a score of 1 should receive a NOAC preferentially over warfarin, whereas the AHA/ACC/HRS guideline recommends no treatment, treatment with aspirin (ASA), or treatment with an oral anticoagulant if the patient wishes. Despite these recommendations, Dr Ruff noted that there are still unresolved questions regarding the incorporation and management of the NOACs into clinical practice.
One unanswered question is the safety of concomitant ASA use in patients with AF who are receiving oral anticoagulation, which is common among patients who also have coronary artery disease (CAD). Rohan Shah, MD, Duke University Medical Center, Durham, North Carolina, USA, presented data from a subanalysis of the ROCKET AF trial.
In the double-blind phase 3 ROCKET AF trial, patients with AF were randomly assigned to receive rivaroxaban or warfarin, with a mean follow-up period of 1.9 years [ROCKET AF Study Investigators. Am Heart J. 2010]. The purpose of this analysis was to determine the safety and efficacy of concomitant ASA and rivaroxaban treatment in patients with AF and CAD.
At baseline, 37% of patients were receiving ASA, of whom 53% remained on ASA during the follow-up period. The mean baseline dose of ASA was 99.2 mg/d. Patients receiving baseline ASA were more likely to have had a prior myocardial infarction (22% vs 14%) and have congestive heart failure (68% vs 59%).
Among patients who were taking ASA at baseline, all-cause mortality, vascular death, and major or nonmajor clinically relevant bleeding occurred more frequently as compared with patients not receiving ASA (Table 1). There was evidence of improved outcomes in patients who received rivaroxaban, and there was no interaction between baseline ASA use and outcomes with rivaroxaban. However, there was a significant interaction between the presence or absence of CAD and the association between baseline ASA and both all-cause mortality and vascular death (Table 2).
Effect of Background ASA on Outcomes in the ROCKET AF Trial
Effect of Background ASA on Outcomes in ROCKET AF Stratified by CAD
Dr Shah acknowledged that a limitation of this analysis was that it was post hoc, that the study included patients already at high risk of SSE, and that there were potentially additional unmeasured cofounders. He stated that although baseline ASA use was associated with increased risk of death and bleeding, particularly for patients without CAD, the results of this analysis should be considered hypothesis generating.
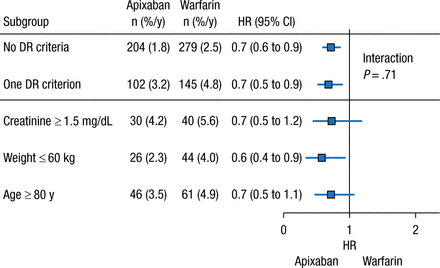
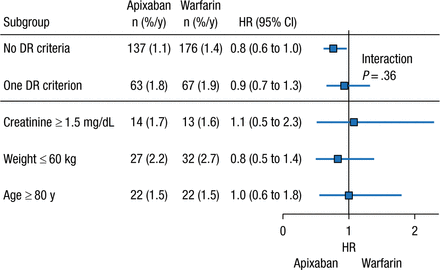
Another unanswered question regarding the NOACs is the role of dose reductions in certain patient populations. In clinical practice, dose reduction of the NOACs occurs more frequently than expected on the basis of the clinical trial criteria, stated John H. Alexander, MD, MHS, Duke University Medical Center, Durham, North Carolina, USA. As a result of the apparent overprescription of reduced-dose apixaban (2.5 mg BID), an analysis of the phase 3 ARISTOTLE trial was performed to determine the safety and efficacy of apixaban 5 mg BID compared with warfarin in patients who did not meet the criteria for a dose reduction but had 1 factor of the dose reduction criteria.
In the ARISTOTLE trial, dose reduction was warranted if patients had ≥ 2 of the following factors: age ≥ 80 years, weight ≤ 60 kg, or creatinine level ≥ 1.5 mg/dL at baseline [Granger CB et al. NEJM. 2011]. The analysis included patients who had 0 or 1 dose reduction criterion who were receiving apixaban 5 mg BID. Among patients receiving apixaban 5 mg BID, 73.9% had 0 factors for dose reduction, whereas 5.0% had higher creatinine clearance, 7.9% had low body weight, and 9.1% were aged ≥ 80 years. Patients with 1 dose reduction criterion had a greater mean age; were more likely to be women; and had higher rates of prior stroke or transient ischemic attack, greater CHADS2 score, history of bleeding, and history of fall, as compared with patients who had none of the criteria.
The presence of ≥ 1 dose reduction factor was associated with greater rates of annual SSE and major bleeding events, regardless of treatment with apixaban or warfarin. However, patients who received apixaban 5 mg BID with 0 or 1 dose reduction criterion experienced similar risk reduction for major bleeding and SSE vs patients who received warfarin (Figures 1 and 2). Given these data, Dr Alexander concluded that patients with only 1 dose reduction criterion, including extremes of these criteria, should receive a 5-mg twice-daily dose of apixaban rather than the reduced dose of 2.5 mg twice daily.
Major Bleeding With Apixaban With ≤ 1 DR Criterion
Values presented as number of patients (percentage per year).
DR, dose reduction.
Reproduced with permission from JH Alexander, MD.
Systemic Embolism Rate With Apixaban With ≤ 1 DR Criterion
Values presented as number of patients (percentage per year).
DR, dose reduction.
Reproduced with permission from JH Alexander, MD.
Kidney function, as measured by creatinine clearance or estimated glomerular filtration rate (eGFR), is an important factor to consider when prescribing a NOAC. Indeed, in most oral anticoagulation trials, patients with an eGFR < 30 mL/min/1.73 m2 were excluded, leading to a paucity of data in this population. Anders Nissen Bonde, MD, Gentofte University Hospital, Gentofte, Denmark, presented data from a cohort study evaluating the effect of kidney impairment on risk of stroke and bleeding in patients with AF.
At baseline, patients with an eGFR between 30 and 15 mL/min/1.73 m2 were most likely to be of older age and have high CHA2DS2-VASc and HAS-BLED scores. In the analysis, the rates of systemic embolism (SE) and major bleeding were stratified by level of eGFR and need for dialysis.
Regardless of warfarin use, rates of SE and major bleeding rose incrementally in patients with lower eGFR. However, rates of SE were generally higher among patients who did not receive warfarin, whereas rates of major bleeding were generally higher among patients who received warfarin. These data suggest that kidney impairment is associated with a greater risk of SE and major bleeding in patients with AF.
Many questions regarding the management of NOACs remain unanswered or require additional research for clearer answers. Jean-Yves Le Heuzey, MD, Hospital Georges Pompidou, Paris, France, described ongoing registries and trials for the NOACs and ongoing research for unmet needs such as valvular AF and the development of reversal agents.
Unanswered questions include the safety and efficacy of the NOACs used in the settings of percutaneous coronary intervention, embolic stroke of undetermined source, catheter ablation, left atrial or atrial appendage thrombus, cardioversion, patients with cancer, subclinical AF, and valvular AF.
In particular, the lack of an antidote or evidence-based reversal strategy for the NOACs has been a topic of ongoing research, with several antidotes in clinical trials. Aripazine (PER977) is a synthetic small molecule that binds to all of the NOACs, and it has been demonstrated to reverse the anticoagulant effect of dabigatran and rivaroxaban in human blood. Andexanet alfa is a factor Xa inhibitor that competitively inhibits apixaban, edoxaban, and rivaroxaban at factor Xa [Bakhru S et al. AHA 2013 (abstr 11395)]. Idarucizumab is a humanized Fab fragment targeted to dabigatran that has about a 350-fold greater affinity than that of thrombin. Recent data indicate that idarucizumab was effective in reversing the anticoagulant effect of dabigatran, as measured by thrombin time, dilute thrombin time, and ecarin clotting time in patients who had serious bleeding or required urgent surgery or intervention [Pollack CV et al. N Engl J Med. 2015].
In conclusion, although the NOACs have demonstrated safety and efficacy among patients with nonvalvular AF, there are still unmet needs and unanswered questions regarding their use. Numerous ongoing registries and clinical trials will serve to answer many of these questions in the near future.
- © 2015 SAGE Publications