Summary
The IMPROVE-IT trial showed that the cholesterol-lowering drug ezetimibe, when added to simvastatin, provides greater improvement in cardiovascular outcomes among patients with diabetes vs those without diabetes in a population with acute coronary syndrome. Additionally, the rate of new-onset diabetes was not increased among patients treated with ezetimibe.
- acute coronary syndrome
- cardiovascular risk
- cholesterol
- diabetes mellitus
- ezetimibe
- IMPROVE-IT
- simvastatin
The IMPROVE-IT trial showed that the cholesterol absorption inhibitor ezetimibe, when added to statin therapy, reduced cardiovascular events in patients with acute coronary syndrome (ACS) [Cannon CP et al. N Engl J Med. 2015]. Among patients with diabetes, ezetimibe has been shown to reduce the levels of low-density lipoprotein cholesterol (LDL-C) and other lipids, and lower insulin resistance. Data from 2 meta-analyses suggested that intensive-dose statin therapy can increase the risk of new-onset diabetes mellitus (NODM) [Preiss D et al. JAMA. 2011; Sattar N et al. Lancet. 2010]. A pooled analysis comparing simvastatin alone vs ezetimibe plus simvastatin in patients without diabetes found both arms had small but significant increases in fasting glucose but there were no between-group differences [Toth P et al. J Am Coll Cardiol. 2015].
In the IMPROVE-IT trial, a total of 18 144 patients stabilized after ACS ≤ 10 days were randomized to ezetimibe 10 mg plus simvastatin 40 mg vs placebo plus simvastatin 40 mg for a minimum 2.5 years. A recent analysis of this trial, presented by Robert P. Giugliano, MD, SM, Brigham and Women’s Hospital, Boston, Massachusetts, USA, examined the effects of ezetimibe vs placebo in a prespecified subgroup of patients with diabetes (n = 4933) vs patients without diabetes (n = 13 202). The primary end point was the composite of cardiovascular death, myocardial infarction (MI), documented unstable angina (UA) requiring rehospitalization, coronary revascularization (≥ 30 days), or stroke.
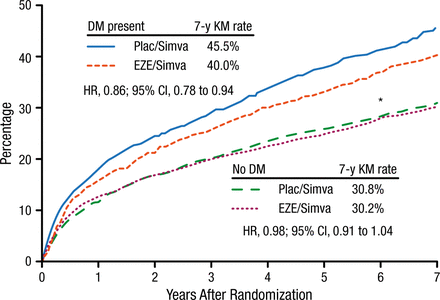
The intention-to-treat results demonstrated a significantly lower risk for the primary end point among patients with diabetes treated with ezetimibe plus simvastatin vs placebo plus simvastatin (40% vs 46%; HR, 0.86; 95% CI, 0.78 to 0.94) after 7 years (Figure 1). Among patients without diabetes, there was no significant difference in the primary end point between the treatment groups. Patients with diabetes had a significantly greater benefit from ezetimibe than those without diabetes (PInt = .023).
Primary Composite End Point at 7 Years After Randomization (Intention-to-Treat)
Cardiovascular death, myocardial infarction, documented unstable angina requiring rehospitalization, coronary revascularization (≥ 30 d), or stroke.
DM, diabetes mellitus; EZE, ezetimibe; KM, Kaplan-Meier; plac, placebo; simva, simvastatin.
*Pint = .023.
Reproduced with permission from RP Giugliano, MD
Analysis of the individual cardiovascular end points in patients with diabetes treated with ezetimibe showed a significant reduction in MI (24% reduction, PInt = .028) and ischemic stroke (39% reduction, PInt = .031) but not in cardiovascular death (PInt = .57), when compared with patients without diabetes who were treated with ezetimibe. There was no significant difference in the safety profile of ezetimibe when stratified by the presence of diabetes.
The IMPROVE-IT trial investigators also analyzed the occurrence of NODM among patients treated with ezetimibe. The results were presented by Michael A. Blazing, MD, Duke Clinical Research Institute, Durham, North Carolina, USA. NODM was defined as the initiation of diabetes medication or 2 consecutive fasting glucose levels ≥ 7 mmol/L. Patients with pre-existing diabetes were excluded from the analysis population. Pre-existing diabetes was defined as use of a hypoglycemic drug or elevated glucose at randomization (fasting glucose ≥ 7 mmol/L or nonfasting glucose ≥ 11.1 mmol/L).
After a mean follow-up of 75 months, a total of 1414 (13.3%) patients were diagnosed with NODM. The risk of NODM was similar between the 2 treatment arms, with 720 patients developing NODM in the ezetimibe plus simvastatin group compared with 694 in the placebo plus simvastatin group (HR, 1.04; 95% CI, 0.94 to 1.15; P = .46).
A breakdown of the primary outcome by the defining criteria for NODM showed similar percentages of NODM in the 2 treatment groups. A sensitivity analysis using 4 alternate NODM definitions and an alternate exclusion definition for prior diabetes was performed. The alternate definitions were as follows: (1) initiation of a hypoglycemic drug; (2) 2 consecutive fasting glucose levels ≥ 7 mmol/L; (3) diabetes-related adverse event reporting; and (4) either no. 1 or 3. The alternate definition for diabetes exclusion at randomization included investigator-reported diabetes in the case report form. These analyses found no significant difference in the numbers of NODM in the ezetimibe plus simvastatin vs the simvastatin groups using any of the alternate definitions.
These analyses of the IMPROVE-IT trial provide new information on the effects of ezetimibe on patients with diabetes and the potential for risk of NODM with the use of ezetimibe. The first analysis showed that patients with diabetes had a higher risk for cardiovascular events than patients without diabetes. This translated to a greater relative and absolute benefit from the addition of ezetimibe to simvastatin in patients with diabetes as compared with patients without diabetes. The increased benefit in patients with diabetes was driven by reductions in MI and ischemic stroke. The safety profile of ezetimibe plus simvastatin was similar to that of placebo plus simvastatin in both diabetes and nondiabetes groups. The second analysis showed that the rates of NODM at 75 months’ follow-up were similar in the groups treated with simvastatin plus ezetimibe and simvastatin plus placebo.
- © 2015 SAGE Publications