Summary
An analysis of mortality in the DAPT study revealed that although there was a borderline increase in mortality in the continued thienopyridine arm, it was mainly related to non-cardiovascular deaths, and bleeding accounted for a minority of these deaths. Although cancer-related deaths were increased with continued thienopyridine, there was no difference in cancer incidence between randomized groups, and these deaths may have been attributed to an imbalance in subjects with known cancer at the time of enrollment, Although dual antiplatelet therapy beyond 12 months after coronary stenting should be considered for prevention of myocardial infarction, the risks should be considered carefully.
- cancer
- bleeding risk
- DAPT Study
- dual antiplatelet therapy
- cardiovascular mortality
- cancer-related death
- thienopyridine
Prolonged dual antiplatelet therapy beyond 1 year was associated with increased mortality in the DAPT study, but whether this was related to the increased bleeding rates seen in the study was unknown [Mauri L et al. N Engl J Med. 2014]. A new analysis has revealed that cancer-related death accounted for the majority of the difference in mortality, and this appeared to be related to an imbalance in advanced cancers enrolled as there was no difference in cancer incidence, according to Laura Mauri, MD, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
The DAPT Study found that dual antiplatelet therapy with a thienopyridine plus aspirin beyond 1 year after coronary stenting, vs aspirin alone, reduced ischemic complications, but increased moderate or severe bleeding in patients treated with drug-eluting stents. The rates of major adverse cardiovascular and cerebrovascular events (MACCE; a composite of death, myocardial infarction [MI], and stroke) and MI were significantly lower in the continued thienopyridine group (n = 5020) compared with the placebo group (n = 4941), at 4.3% vs 5.9%, and 2.1% vs 4.1%, respectively (both P < .001); but the rate of all-cause death was higher in the continued thienopyridine group (2.0% vs 1.5%; P = .05).
The objective of the present analysis was to adjudicate and analyze deaths following randomization for all subjects (treated with either drug-eluting or bare metal stents), with particular focus on bleeding- and cancer-related outcomes. A total of 11 648 patients were randomized (5862 to continued thienopyridine vs 5786 to placebo).
There was a trend toward increased all-cause mortality for the 12- to 30-month period at 1.9% in the continued thienopyridine group vs 1.5% in the placebo group (HR, 1.31; 95% CI, 0.97 to 1.75; P = .07). It reached statistical significance for the 12- to 33-month period at 2.2% vs 1.8% (HR, 1.32; 95% CI, 1.00 to 1.73; P = .05). In the continued thienopyridine group, non-cardiovascular (CV) death was more frequent during the 12- to 30-month period (0.9% vs 0.5%; HR, 1.94; 95% CI, 1.20 to 3.15; P = .01), whereas CV death was numerically more frequent during the 30- to 33-month period, as thienopyridine treatment was discontinued (0.3% vs 0.1%; HR, 2.39; 95% CI, 0.84 to 6.77; P = .09).
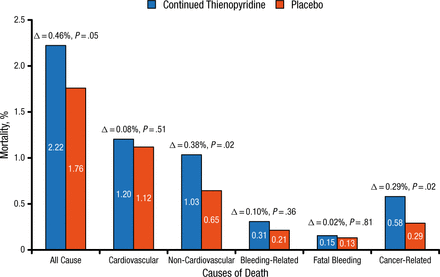
For the entire 12- to 33-month period, non-CV deaths and cancer-related deaths were significantly higher in the continued thienopyridine group compared with the placebo group, and the rate of bleeding-related deaths was very low and did not differ significantly between the groups (Figure 1). However, there was no difference in new cancer incidence after randomization. Most of these cancer-related deaths were solid tumors typical of such a population, rather than a particular location or cell type, and cancer deaths were rarely related to bleeding. Although life expectancy of less than 3 years was an exclusion criterion of the study, patients with cancer were allowed to be enrolled. These findings suggest that caution is warranted in choosing whether to continue long-term dual antiplatelet therapy in subjects with advanced cancer. There have been no observed increases in mortality [Elmariah S et al. Lancet. 2015] or cancer-related death [Hicks BM et al. Pharmacoepidemiol Drug Saf. 2015; Unger EF. N Engl J Med. 2009; Roe MT et al. N Engl J Med. 2012] across prior large randomized trials of thienopyridine therapy with extended follow-up.
Mortality by Cause
12-33 months; n = 11 648 randomized.
Reproduced with permission from L Mauri, MD.
Dr Mauri cautioned that there were several limitations to the study, including low event rates and the retrospective adjudication of cancer diagnosis. She concluded that dual antiplatelet therapy beyond 12 months after coronary stenting should be considered for the prevention of MI, but risks of continued dual antiplatelet therapy should be considered carefully.
- © 2015 SAGE Publications