Summary
Secondary analysis of a small pilot study has shown that botulinum toxin vs placebo reduces the burden of atrial fibrillation at 1 year after coronary artery bypass graft surgery. An improvement in heart rate variability also suggests that botulinum toxin injected into fat pads is a neuromodulator with effects on the autonomic nervous system.
- atrial fibrillation
- autonomic nervous system
- botulinum toxin
- coronary artery bypass graft surgery
- CABG
- neuromodulator
- implantable loop recorder
- arrhythmias
- cardiology & cardiovascular medicine clinical trials
Botulinum toxin injected into the epicardial fat pads during coronary artery bypass graft (CABG) surgery significantly reduced the primary end point of atrial fibrillation (AF) events by 10% compared with placebo (P = .024) at 30 days in a randomized pilot study [Pokushalov E et al. J Am Coll Cardiol. 2014]. New results from a secondary analysis showed this benefit extended to 1 year, along with reduction in heart rate variability (HRV), according to Jonathan S. Steinberg, MD, The University of Rochester, Rochester, New York, and The Valley Health System, New York, New York, USA.
The prospective, double-blind study was conducted in 2 centers and randomized 30 patients to botulinum toxin (type A; 50 U/1 mL per fat pad) and 30 patients to placebo (normal saline; 1 mL per fat pad). The patients were mostly men (about 80%) who were aged about 62 years and had a history of paroxysmal AF and indication for CABG using the American College of Cardiology/American Heart Association guidelines. An implantable loop recorder was placed on the day of surgery in all patients to capture AF events and burden, and data were collected at 3, 6, 9, and 12 months. Holter recordings were used to obtain serial measurements of HRV to assess the effect of botulinum toxin on the autonomic nervous system, which plays a key role in the initiation and maintenance of AF. This toxin is known to interfere with neurotransmitter release.
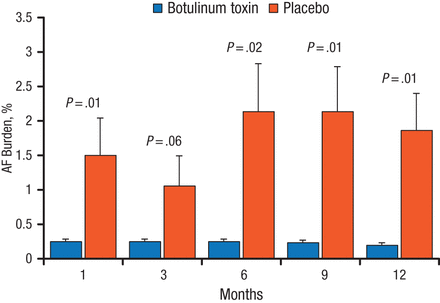
In the secondary analysis, there was a marked and significant reduction in AF burden with botulinum toxin vs placebo at all assessments except 3 months (Figure 1). All patients would be considered responders, stated Dr Steinberg, because they did not meet the conventional implantable loop recorder criteria for AF burden of > .5% per month. In the placebo group, 6 patients required additional drug therapy because of clinically frequent AF, and 2 patients required catheter ablation for persistent AF; there were none in the botulinum toxin group. No strokes or other serious clinical events occurred in either group.
AF Burden With 1-Year Follow-up
AF, atrial fibrillation.
Reprinted with permission from JS Steinberg, MD.
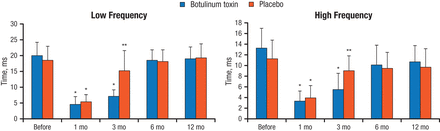
HRV, measured as standard deviation of the NN intervals, was reduced substantially in both groups at 1 month after surgery (P < .05 vs baseline). With botulinum toxin, HRV was significantly lower at 3 months vs baseline (P < .05) and then recovered to near-baseline levels, while HRV recovered to near-baseline levels at 3 months in the placebo group and was maintained. The frequency of HRV in the patients with low and with high frequency was reduced early after surgery with botulinum toxin and placebo, with a similar pattern of rebounding to baseline levels (Figure 2).
Frequency of Heart Rate Variability
*P < .05 vs baseline; **P < .05 between groups.
Reprinted with permission from JS Steinberg, MD.
According to Dr Steinberg, the alterations in HRV with botulinum toxin suggest there were reductions in parasympathetic and sympathetic activity, but the changes dissipated between 3 and 6 months as expected. Among the limitations of this study are the small number of patients, the lack of data on AF burden prior to surgery, no objective testing to confirm the denervation effect, and no confirmation of functional atrial remodeling or its mechanisms. Although these data suggest botulinum toxin may be a neuromodulator, large-scale trials are required to evaluate its possible value to reduce postoperative AF and in other clinical settings.
- © 2015 SAGE Publications