Summary
A shifting scientific paradigm in disease understanding and management is leading to a systems approach where diseases are modeled in an interactome that consolidates all molecular interactions within a cell. This approach can lead to a systematic understanding of the molecular mechanisms underlying a complex disease and aid in successful treatment.
- cardiovascular clinical research
- disease module
- interactome
- mobile technologies
- systems approach
- genomics
- cardiology & cardiovascular medicine clinical trials
Joseph Loscalzo, MD, PhD, Brigham and Women’s Hospital, Boston, Massachusetts, USA, focused on systems and networks in biomedicine and the importance of these topics in drug development, while Robert A. Harrington, MD, Stanford University, Stanford, California, USA, presented a broader picture of the current challenges and opportunities in cardiovascular (CV) clinical research.
Dr Loscalzo started by hypothesizing that the conventional reductionist scientific approach of addressing 1 variable at a time might be too simplistic in the context of disease understanding and management. The changing scientific paradigm suggests that most biological systems respond to multiple inputs that can vary and interact. Such systems are complex and involve molecular networks that can be modeled using novel quantitative approaches. This method can help to identify changes that may lead to a disease and examine responses to drug-induced perturbations in the networks.
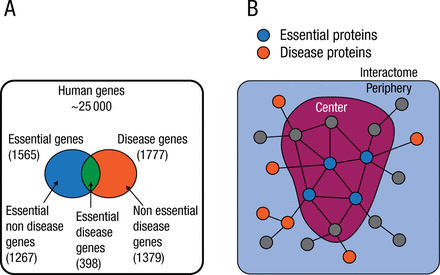
The conventional pathophenotype classification of diseases also has multiple shortcomings. There is genetic overlap among common complex human pathophenotypes, and different chronic diseases have common genome-wide association study loci [Rzhetsky A et al. Proc Natl Acad Sci USA. 2007; Wellcome Trust Case Control Consortium. Nature. 2007]. Most chronic diseases rarely result from an abnormality in a single gene but rather are a consequence of the interplay of multiple molecular processes. The relationships between these processes can be represented in an interactome, a network that consolidates all physical protein–protein interactions within a cell (Figure 1) [Barabási AL et al. Nat Rev Genet. 2011].
The Interactome: Disease and Essential Genes
Adapted by permission from Macmillan Publishers Ltd: Nat Rev Genet. Vol 12, Barabási AL et al, Network Medicine: A Network-based Approach to Human Disease, Pages 56-68. Copyright (2011).
Dr Loscalzo presented a recent study that developed a mathematical model for the identification of disease modules and showed that the location of each disease module in the network determined its pathobiological relationship to other diseases [Menche J et al. Science. 2015].
Disease-associated proteins tend to interact with each other and cluster in the same neighborhood of the interactome, forming a disease module. The accurate identification of a disease module can lead to a systematic understanding of the molecular mechanisms underlying a complex disease.
Despite advances in disease gene identification and high-throughput interactome mapping, the interactome remains incomplete, and the lack of information leaves many disease proteins isolated from their disease module. The interactome-based model developed in the study helps to predict molecular commonalities between phenotypically related diseases, even when they do not share primary disease genes.
The study included 299 diseases defined by Medical Subject Headings ontology that have at least 20 associated genes in the current Online Mendelian Inheritance in Man and genome-wide association study databases and 2436 disease-associated proteins.
The findings showed that if a disease is highly clustered topologically, it suggests greater functional similarity of the genes in the disease module. Diseases with overlapping modules have significant molecular similarity, elevated coexpression of their associated genes, as well as similar symptoms and high comorbidity. Not surprisingly, coronary artery disease and atherosclerosis were found to overlap. However, the model also predicted overlapping disease modules of seemingly unrelated conditions, such as asthma and celiac disease. A closer look revealed that the immunoglobulin A production pathway plays a biological role in both of these diseases.
Dr Loscalzo speculated that the main reason for declining successful drug development is the reductionist goal to identify a single target with a single “magic bullet” drug. Such drug targets are usually approached in isolation from the disease module, although it may be more effective to characterize a drug target with regard to its effects on the phenotype. Some combination treatments are already using the approach of targeting the pathway and succeed in treating a disease in situations where a single treatment often fails because of emerging drug resistance [Flaherty KT et al. N Engl J Med. 2012]. Dr Loscalzo concluded by suggesting that interactomes can also help to identify existing drugs that can be repurposed to treat a different disease.
Dr Harrington focused on larger economic and scientific issues affecting CV clinical research in the United States. One of the pressing challenges in CV clinical research has been the increasing operational complexity and expense of performing studies in the United States [Antman EM, Harrington RA. JAMA. 2012]. CV randomized controlled trials sponsored by the National Heart, Lung, and Blood Institute have substantial rates of international enrollment [Kim ES et al. J Am Coll Cardiol. 2011], and the percentage of US participants in global clinical trials has been steadily declining over the past 20 years in Dr Harrington’s observation.
While the cost of clinical research has been increasing, the rate of allocated federal funding has remained the same over the past 2 decades [Dorsey ER et al. JAMA. 2010]. As a result, researchers have to rely more heavily on industry funding, leading to a closer and more complicated relationship with the industry. Limited government funding also resulted in a smaller percentage of funded R01 grant applications and the higher average age of a first-time R01 recipient [Larson RC et al. Serv Sci. 2012]. Private investors are also more interested in targeted treatments for rare cancers and orphan diseases that have a potential for high pricing, while other therapeutic areas do not receive as much investor interest despite their high prevalence [Kocher R, Roberts B. N Engl J Med. 2014].
Possible solutions presented by Dr Harrington included increasing federal funding while also maximizing the scientific yield of each study. Some scientists have favored moving away from traditional phase 1 testing and embracing new approaches to drug development [Loscalzo J. Circulation. 2012]. Echoing Dr Loscalzo’s presentation, Dr Harrington highlighted the importance of genomics, proteomics, metabolomics, pathophenomics, and systems pharmacology.
Other approaches in increasing the yield of each study can focus on simplifying clinical trials and creating access to aggregate patient data by introducing randomization into medical society registries [Antman E, Harrington RA. JAMA. 2012]. This step would allow access to a wealth of patient information at a significantly lower cost than performing a study.
Engaging patients via social media and mobile technologies can also substantially increase patient enrollment in a very short period of time. For example, Stanford’s ResearchKit app gained 11 000 participants in just 24 hours, while approximately 1 year is usually required to enroll that many patients in a typical clinical trial.
Dr Harrington concluded by highlighting the need for better investment in the next generation of clinical researchers by providing career coaching and training in CV research and the new tools available for processing large quantities of data.
- © 2015 SAGE Publications