Summary
This article discusses vagal stimulation as a treatment for chronic heart failure (CHF), an overview of research on baroreceptor stimulation, renal denervation in the treatment of HF, as well as the use of spinal cord stimulation to prevent ventricular tachycardia or fibrillation and sudden cardiac arrest.
- Heart Failure
- Interventional Techniques & Devices
- Cardiology & Cardiovascular Medicine
- Heart Failure
- Interventional Techniques & Devices
Jurgen Kuschyk, MD, University Hospital Heidelberg, Heidelberg, Germany, discussed vagal stimulation as a treatment for chronic heart failure (CHF). Autonomic modulation for heart failure (HF) can be achieved through stimulation of the vagus nerve, spinal cord, or carotid baroreceptor; renal nerve ablation; and pharmacologic inhibition of the beta-adrenergic receptor or If channel. The approaches target afferents (arterial chemoreceptors or baroreceptors, and muscle metaboreceptors) or efferents (parasympathetic or sympathetic nerves).
Vagal or spinal cord stimulation affects parasympathetic and sympathetic nerves, and it is rooted in a technology developed almost 50 years ago. The selective stimulation approach is based on the knowledge that increased sympathetic activity and reduced vagal activity are linked with increased mortality following myocardial infarction (MI) and in HF. Increased parasympathetic nerve activity by vagal stimulation has been associated with reduced mortality in animal models of post-MI sudden cardiac death [Vanoli E et al. Circ Res 1991] and CHF [Hamann JJ et al. Eur J Heart Fail 2013], decreased interstitial fibrosis, and increased capillary density [Sabbah HN. Clev Clin J Med 2011]. Proposed benefits include heart rate control, increased variability of heart rate, alterations in expression of nitric oxide and cytokine, and antiarrhythmic effects [Olshansky B et al. Circulation 2008].
A multicenter, Phase 2, European pilot study examined the safety and efficacy of right vagus nerve stimulation using the CardioFit device. The device is an implantable vagal neurostimulator system delivering low-current electrical pulses. It is designed to sense heart rate and to deliver stimulation at a variable delay. The study involved 32 CHF patients (30 males and 2 females), aged 18 to 75 years of age, with New York Heart Association (NYHA) functional Class II to IV, and with a mean heart rate of 60 to 110 bpm and left ventricular ejection fraction (LVEF) ≤35% [De Ferrari GM et al. Eur Heart J 2011]. The primary end points were device safety and side effects. Twenty-three of the patients were followed up with for 12 months. The implant success rate was 100% with only 2 serious adverse events (pulmonary edema and a loose screw). Twelve patients experienced side effects that were resolved. The device was associated with an improvement in NYHA class, and additional studies of the device are ongoing.
Heart rate was reduced from 81.9 bpm at baseline to 75.1 at 3 months (p=0.014) and 76.0 at 6 months (p=0.038). Improvements were also evident in LVEF (22%, 26%, and 29% at baseline, 3 months [p=0.004], and 6 months [p=0.0001], respectively), and in both quality of life and exercise tolerance.
The Increase of Vagal Tone in Chronic Heart Failure [INOVATE-HF: NCT01303718] randomized trial that evaluated the safety and efficacy of vagal stimulation in 650 patients with HF and LV dysfunction supports the use of this technique as an adjunct therapy.
Douglas P. Zipes, MD, Indiana University School of Medicine, Indianapolis, Indiana, USA, discussed the use of spinal cord stimulation to prevent ventricular tachycardia or fibrillation and sudden cardiac arrest. Two neural manipulations can influence cardiac arrhythmias: blockade or interruption of the arrhythmogenic limb (usually, the sympathetic) and stimulation of the antiarrhythmogenic limb (usually, the vagus).
Spinal cord stimulation as a means of relief from the pain of intractable angina pectoris was first studied nearly 30 years ago. Angina pectoris relief by the electrical stimulation of the carotid-sinus nerves was first reported more than 50 years ago. The vagal enhancement that can be attained by spinal cord stimulation inhibits sympathetic excitation and release of norepinephrine, reduces inflammation, modulates nitric oxide, and increases potassium currents. A study in a dog model of ischemic ventricular arrhythmia reported more arrhythmias in the absence of spinal cord stimulation [Issa ZF et al. Circulation 2005].
These and other studies have provided the backdrop to the ongoing Determining the Feasibility of Spinal Cord Neuromodulation for the Treatment of Chronic Heart Failure [DEFEAT-HF; NCT01112579] prospective, multicenter, randomized, parallel-controlled study, in which ranges of stimulus amplitude, pulse duration, and frequency were applied for 6 to 24 hours daily. Inclusion criteria included LVEF ≤35%, NYHA functional Class III, and QRS duration <120 ms. The primary outcome measure of the study is the change in left ventricular volumes as measured by cardiac echo. If successful (results are anticipated in the fall of 2014), spinal cord stimulation will be supported as a therapy to reduce ventricular arrhythmias and improve left ventricular function.
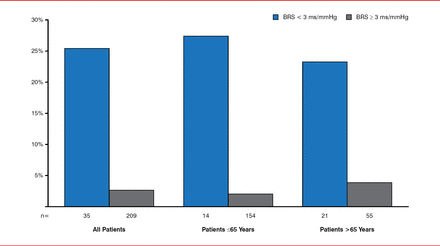
Gaetano De Ferrari, MD, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, provided an overview of research on baroreceptor stimulation. An animal model of MI developed more than 25 years ago provided evidence that increased baroreflex sensitivity was associated with a reduction in the risk of ventricular fibrillation. An observational study in 1300 patients with diabetes and recent MI demonstrated similar findings, and a contemporary study found that subjects with depressed baroreceptor sensitivity were at much greater risk of death than patients with preserved baroreceptor sensitivity (Figure 1) [De Ferrari GM et al. J Am Coll Cardiol 2007].
Baroreflexes and Long-Term Mortality Following Myocardial Infarction
Reproduced from De Ferrari GM et al. Baroreflex sensitivity predicts long-term cardiovascular mortality after myocardial infarction even in patients with preserved left ventricular function. J Am Coll Cardiol 2007;50(24):2285–2290. With permission from Elsevier.
The situation is similar for patients with chronic congestive heart failure. Carotid sinus nerves can be stimulated with an implanted electrode. Animal models of heart failure have indicated the potential value of this approach [Sabbah HN et al. Circ Heart Fail 2011], and clinical studies have suggested the efficacy of baroreceptor stimulation in patients with drug-resistant hypertension [Bakris GL et al. J Am Soc Hypertens 2012]. A very preliminary pilot study in patients with heart failure has been promising, showing a significant and sustained reduction in muscle sympathetic nerve activity, and larger Phase 2 trials are currently ongoing.
Finally, Dominik K. Linz, MD, PhD, University Hospital of Saarland, Homburg/Saar, Germany, discussed renal denervation in the treatment of HF. Increased central sympathetic afferent and efferent activity is involved in pathologies, including diabetes, metabolic syndrome, hypertension, chronic kidney disease, obstructive sleep apnea, congestive heart failure, and arrhythmias. Interrupting the cross-talk between the heart and other sympathetic organs may be efficacious. Among the strategies is renal sympathetic denervation done using a catheter inserted through the aorta that supplies high-frequency energy. The approach can reduce renal spillover of norepinephrine by nearly 50% [Schlaich MP et al. N Engl J Med 2009] and muscle sympathetic nerve activity by almost 40% at 6 months following denervation [Hering D et al. Circulation 2013]. Although renal denervation was effective in reducing diastolic and systolic blood pressure for up to 3 years in the SYMPLICITY HTN-1 and −2 trials [Schlaich MP. TCT 2012], it was not associated with significant changes in blood pressure in the randomized, blinded, sham-controlled SYMPLICITY HTN-3 [Bhatt DL et al. N Engl J Med 2014]
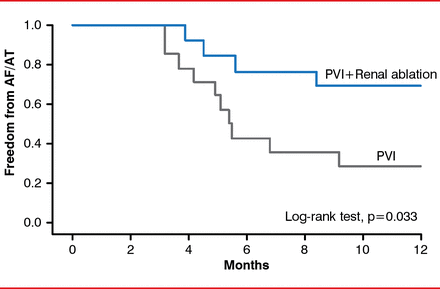
Another study with hypertensive patients reported a benefit for renal denervation in reducing heart rate and AV conduction velocity at 3 and 6 months [Ukena C et al. Int J Cardiol 2012]. Renal denervation provided concomitantly with pulmonary vein isolation was significantly more effective in providing relief from atrial fibrillation and atrial tachycardia in hypertensive patients with treatment-resistant paroxysmal or persistent atrial fibrillation (Figure 2) [Pokushalov E et al. J Am Coll Cardiol 2012].
Pulmonary Vein Isolation With and Without Concomitant Renal Denervation
AF=atrial fibrillation; AT=atrial tachycardia; PVI=pulmonary vein isolation.
Reproduced from Pokushalov E et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol 2012;60(13):1163–1170. With permission from Elsevier.
The ongoing Renal Denervation in Patients With Chronic Heart Failure multicenter trial [RE-ADAPT-CHF; NCT02085668] has enrolled 100 patients with CHF (NYHA Class II to III) and LVEF <40%, and it will assess the safety of renal denervation with special consideration of clinically significant periprocedural adverse events in CHF patients. They are to be followed up with for 12 to 36 months. Although this small study will help inform the research on the effect of renal denervation on arrhythmias and CHF, larger and more rigorous clinical trials need to be performed to more conclusively determine the efficacy of this promising technology.
- © 2014 MD Conference Express®