Summary
Atrial fibrillation (AF) increases the risk of stroke, and this risk is further increased in a linear fashion with higher CHADS2 scores (representing congestive heart failure; hypertension; age =75 years; diabetes mellitus; and prior stroke, transient ischemic attack, or thromboembolism) [Crandall ME et al. Pacing Clin Electrophysiol 2009]. This article discusses catheter ablation (CA) to lower the risk of stroke in AF patients, echocardiographic contraindications for cardioversion, and the use of remote device monitoring to reduce stroke risk.
- Interventional Techniques & Devices
- Arrhythmias
- Cerebrovascular Disease
- Interventional Techniques & Devices
- Cardiology & Cardiovascular Medicine
- Arrhythmias
- Cerebrovascular Disease
Atrial fibrillation (AF) increases the risk of stroke, and this risk is further increased in a linear fashion with higher CHADS2 scores (representing congestive heart failure; hypertension; age ≥75 years; diabetes mellitus; and prior stroke, transient ischemic attack, or thromboembolism) [Crandall ME et al. Pacing Clin Electrophysiol 2009]. The short- and long-term risks of stroke are lower in patients with AF who are treated with catheter ablation (CA), regardless of whether they receive anticoagulation (AC) therapy, stated John D. Day, MD, Intermountain Medical Center, Salt Lake City, Utah, USA.
Dr. Day presented an update on contemporary issues in the management of AF, including the role of CA. Although AC has been shown to reduce stroke risk, even in patients with coronary artery disease plus AF [Wyse DG et al. N Engl J Med 2002], it is also associated with cerebral microbleeds (CMBs). Dr. Day raised his concern that long-term use of AC and the resulting CMBs may increase the long-term risk of developing dementia in these patients.
CMBs are more likely to occur with increased age and in patients on antiplatelet and AC therapy [Yates PA et al. Front Neurol 2014]. The annual risk of CMBs in patients on warfarin therapy was 1% to 10% among 6 studies [Charidimou A et al. Front Neurol 2012], and there is a paucity of data available regarding an association between CMBs and novel oral ACs (NOACs).
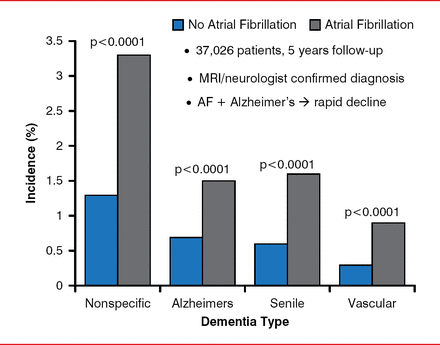
In a longitudinal, observational study, patients with AF were found to have a higher risk of all forms of dementia compared with patients without AF (Figure 1) [Bunch TJ et al. Heart Rhythm 2010]. Moreover, the risk was much higher in patients aged <70 years (OR, 2.5 vs >70 years). A meta-analysis of 8 studies with 77,668 patients (15% with AF) and a mean follow-up of 7.7 years confirmed the higher risk of dementia in relation to AF (HR, 1.42; 95% CI, 1.17 to 1.72; p<0.001) [Santangeli P et al. Heart Rhythm 2012].
Atrial Fibrillation-Increased Risk of Dementia
AF=atrial fibrillation; MRI=magnetic resonance imaging.
Reproduced from Bunch TJ et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer's dementia. Heart Rhythm 2010;7(4)433–437. With permission from Elsevier.
A single-center study with 37,908 patients showed that incidences of mortality, stroke, and dementia were lower for patients with AF who underwent CA compared with those treated medically or those who did not have AF [Bunch TJ et al. J Cardiovasc Electrophysiol 2011]. Alzheimer's dementia developed in 0.2% of the CA-treated patients, compared with 0.9% of patients who received AF but no CA and 0.5% of no-AF patients (p<0.0001). These results are similar to others that have been presented, said Dr. Day, and confirmatory data are awaited from the Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation trial [CABANA; NCT00911508] and Early Atrial Fibrillation Stroke Prevention Trial [EAST; NCT01288352].
ECHOCARDIOGRAPHIC CONTRAINDICATIONS FOR CARDIOVERSION
The identification of left atrial (LA) or LA appendage (LAA) thrombus by transesophageal echocardiography (TEE) is a contraindication for elective transthoracic electrical cardioversion (TEC) in patients with AF because of the risk of a thromboembolic event (TE), primarily stroke [Fuster V et al. Europace 2006].
Thrombus is defined as an echodense lesion that is present in multiple imaging planes and is clearly distinguishable from trabeculation and atrial endocardium. A thrombus is detected by TEE in 10% to 15% of patients with AF, and <1% of these patients have a TE after cardioversion. Ali Oto, MD, Hacettepe University, Ankara, Turkey, noted that patients with a left atrial thrombus should be treated with anticoagulation for 3 to 4 weeks and then have a repeat TEE examination to determine suitability for TEC.
The presence of sludge on TEE, which is defined as a dynamic and gelatinous but not solid or well-formed echodensity that is present throughout the entire cardiac cycle [Troughton RW et al. Heart 2003], is a situation in which the physician must decide how to respond, because no studies have been conducted to determine the safety of TEC in this population. A retrospective 10-year survey of 2705 patients with AF who underwent TEE showed that the prevalence of LA or LAA sludge was approximately 2% [Yarmohammadi H et al. J Am Soc Echocardiogr 2012]. Furthermore, this study found no difference in the rate of TE in patients with LA or LAA thrombus or sludge as compared to patients without LA or LAA thrombus or sludge (about 4% in both groups).
Another finding on TEE, spontaneous echo contrast (SEC), is not a contraindication for TEC, but patients should receive prior therapeutic anticoagulation if possible, according to a review of 9 clinical studies [Patel SV, Flaker G. Clin Cardiol 2008]. SEC is defined as a swirl or smoke that is associated with low blood flow velocity [Troughton RW et al. Heart 2003], and it has 4 grades depending on its intensity, its location, and the presence of swirling movement [Patel SV et al. Clin Cardiol 2007].
REMOTE DEVICE MONITORING TO REDUCE STROKE RISK
Atrial arrhythmias recorded by cardiac implantable electronic devices (CIEDs) are an independent predictor of total mortality, stroke, and chronic AF [Glotzer TV et al. Circulation 2003]. Atrial high rate episodes (AHREs) were recorded in the memory of ∼50% of CIEDs during the battery life span in this study, and most were asymptomatic. The level of AF burden that increases risk appears to vary among studies, ranging from 3.8 hours per day, associated with a 2.0% rate of TE (p=0.006) [Shanmugam N et al. Europace 2012], to ≥5.5 hours daily, associated with a 2.4% of TE (HR, 2.20; 95% CI, 0.96–5.05; p=0.06) [Glotzer TV et al. Circ Arrhythm Electrophysiol 2009]. Device-detected AHREs were associated with an increased risk of TE and stroke, regardless of the predevice history of AF [Shanmugam N et al. Europace 2012].
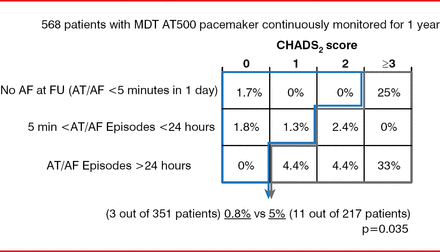
A risk stratification schema created by combining device-detected AHREs and clinical data provides another approach to assist in defining patients at low or high risk for stroke (Figure 2) [Botto GL et al. J Cardiovasc Electrophysiol 2009]. Yet refinement of this schema with additional evidence is needed to determine when anticoagulation should be initiated, stated Renato P. Ricci, MD, S. Filippo Neri Hospital, Rome, Italy. After anticoagulation is initiated, it should not be discontinued even in the absence of device-detected AF.
Risk Stratification Schema Using Clinical Data and Device-Detected Events
AF=atrial fibrillation; AT=atrial tachycardia; FU=follow-up; MDT=Medtronic.
Reproduced from Botto GL et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol 2009;20(3):241–248. With permission from John Wiley and Sons.
Remote monitoring (RM) of CIEDs is being investigated to reduce TE risk. Only one randomized study has been reported, however, which was terminated early due to futility. The Combined Use of BIOTRONIK Home Monitoring and Predefined Anticoagulation to Reduce Stroke Risk study [IMPACT; NCT00559988] showed no difference at 5 years in the primary end point of first stroke, systemic embolism, or major bleed between the RM and usual-treatment patients (2.4 vs 2.3 event rate, respectively; HR, 1.06; p=0.732). The rate of major bleeding was 1.2 and 1.6, respectively, in the control (n=1361) and RM (n=1357) groups, compared with 0.1 for hemorrhagic stroke in both groups, noted Prof. Ricci. In the RM group, only 4.5% of patients who had appropriate anticoagulation (an international normalized ratio [INR] of 2 to 3) had an ischemic stroke, compared with 95.5% of those with an INR <2. This low stroke event rate may have limited the ability to detect a difference with remote monitoring, said Prof. Ricci. Furthermore, device-detected AF was found in only 35% to 50% of patients prior to stroke, and this was within 30 days of the stroke in only 8% to 22% of patients [Brambatti M et al. Circulation 2014; Daoud EG et al. Heart Rhythm 2011]; thus, this temporal dissociation is an emerging issue that must be explored.
- © 2014 MD Conference Express®