Summary
Implantation of a leadless cardiac pacemaker (LCP) offers the potential to eliminate the need for the pocket, generator, and connections in most pacemaker systems—the transvenous lead subcutaneous pocket, subcutaneous pulse generator, and intra-system connections. This article discusses a prospective, nonrandomized Evaluation of a New Cardiac Pacemaker study [LEADLESS; Reddy VY et al. Circulation 2014], which found that permanent leadless cardiac pacing is safe and feasible at 1 year after implantation in patients with an indication for single-chamber (ventricular) pacing.
- Arrhythmias
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
- Arrhythmias
- Interventional Techniques & Devices
- Cardiology Clinical Trials
Implantation of a leadless cardiac pacemaker (LCP) offers the potential to eliminate the need for the pocket, generator, and connections in most pacemaker systems—the transvenous lead subcutaneous pocket, subcutaneous pulse generator, and intra-system connections. Vivek Y. Reddy, MD, Mount Sinai School of Medicine, New York, New York, USA, reported that permanent leadless cardiac pacing is safe and feasible at 1 year after implantation in patients with an indication for single-chamber (ventricular) pacing. The leadless cardiac pacemaker contains a pulse generator and sensing or pacing electrodes within a single, miniaturized unit.
In this prospective, nonrandomized Evaluation of a New Cardiac Pacemaker study [LEADLESS; Reddy VY et al. Circulation 2014], 33 patients received the Nanostim LCP. The device was delivered to the right ventricle using a deflectable delivery catheter and affixed to the myocardium using a distal single-turn (screwin) steroid-eluting helix. The mean age of the patients was 77±8 years, and 67% were male (n=22). Permanent atrial fibrillation with atrioventricular block was the most common reason for cardiac pacing (n=22; 67%). The mean procedure duration was 28±17 minutes, and the average time to hospital discharge was 31±20 hours. The overall complication-free rate was 94% (n=31). Five patients (15%) required the use of more than 1 leadless cardiac pacemaker during the procedure. One male patient sustained right ventricular perforation and cardiac tamponade during implantation; although this was successfully surgically repaired, he ultimately died approximately 1 week later from an AF-related stroke. The implant success rate was 97% (32/33).
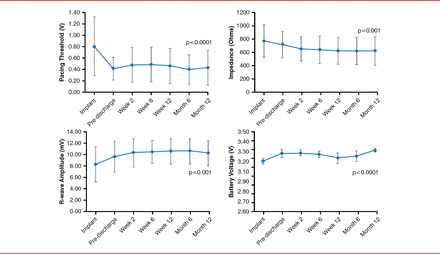
After 1 year, the measures of pacing performance (sensing, impedance, and pacing threshold) either improved or were stable within the accepted range. Pacing threshold was 0.43±0.30 V (p<0.0001), R-wave amplitude was 10.32±2.23 mV (p=0.001), impedance was 627±209 ohms (p=0.001), and battery voltage was 3.29±0.02 V (p<0.0001). The p values were derived from a comparison between that measured at 12 months and at time of implantation (Figure 1).
Pacing and Sensing Parameters
Reproduced with permission from VY Reddy, MD.
There were no device migration or dislodgements, no infections, no mechanical failure or early battery depletion, and no pro-arrhythmias detected after 1 year.
A completely self-contained, single-chamber, leadless cardiac pacemaker is stable and highly reliable over a 1-year time frame, with few safety issues. This small study suggests the leadless pacemaker could represent a paradigm shift in cardiac pacing. Future studies include a USA multicenter, prospective, single-arm FDA study of ∼667 patients indicated for ventricular pacing and rate-adaptive therapy, as well as a European observational study (both studies are in progress). Also in development is an atrial LCP that will perform dual- or multi-chamber pacing.
- © 2014 MD Conference Express®