Summary
The BOLERO-1 phase 3 clinical trial demonstrated that adding everolimus to weekly trastuzumab and paclitaxel, as first-line therapy, did not improve progression-free survival in women with previously untreated human epidermal growth factor receptor-2-positive advanced breast cancer. PFS was increased by 7 months with everolimus therapy in the HR-negative subgroup; however, this did not cross the statistical significance threshold. Increased adverse events were also reported in the everolimus-treated group, as well as on treatment, AE-related deaths.
- combination treatment
- progression-free survival
- metastatic breast cancer
- locally advanced breast cancer
- everolimus
- trastuzumab
- paclitaxel
- Everolimus in Combination With Trastuzumab and Paclitaxel in the Treatment of HER2 Positive Locally Advanced or Metastatic Breast Cancer
- BOLERO-1
- NCT00876395
Sara A. Hurvitz, MD, University of California at Los Angeles, Los Angeles, California, USA, shared data from the Everolimus in Combination With Trastuzumab and Paclitaxel in the Treatment of HER2 Positive Locally Advanced or Metastatic Breast Cancer trial [BOLERO-1; NCT00876395]. The data showed that adding everolimus to combination trastuzumab and paclitaxel therapy did not improve progression-free survival (PFS) in women with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (BC).
Resistance to trastuzumab remains a significant challenge in the treatment of HER2-positive BC, said Dr Hurvitz, adding that hyperactivation of the phosphoinositide-3 kinase/mammalian target of rapamycin (mTOR) pathway has been implicated in this resistance [Hurvitz SA et al. Cancer Treat Rev. 2013], and mTOR inhibitors have shown some potential to increase PFS in this patient population [André F et al. Lancet Oncol. 2014].
The phase 3 BOLERO-1 trial was subsequently conducted in patients (n = 719) with locally advanced or metastatic HER2-positive BC who had received no prior therapy (other than endocrine therapy, prior adjuvant or neoadjuvant trastuzumab therapy, or chemotherapy). Participants were randomized 2:1 to receive everolimus (10 mg PO daily) plus weekly paclitaxel and trastuzumab, or placebo plus weekly paclitaxel and trastuzumab. Treatment continued to the point of disease progression or intolerable toxicity.
The primary end point was PFS in the entire study population and in a hormone receptor–negative subgroup of patients. Secondary end points included overall response rate (ORR), clinical benefit rate (CBR), and safety.
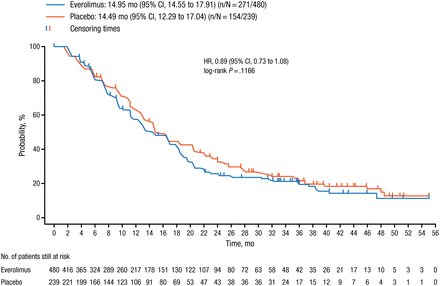
At the time of the final analysis, performed after 425 PFS events in the entire study population, there was no significant difference in median PFS between the everolimus and placebo arms (14.95 vs 14.49 months; HR, 0.89; 95% CI, 0.73 to 1.08; log-rank P = .1166; Figure 1).
Progression-Free Survival in the BOLERO-1 Entire Study Population
One-sided P value is obtained from the log-rank test stratified by prior use of trastuzumab (yes/no) and visceral metastasis (yes/no) from Interactive Web Response System.
Reproduced with permission from SA Hurvitz, MD.
PFS was increased with everolimus therapy in the hormone receptor-negative subgroup (20.27 vs 13.08 months; HR, 0.66; log-rank P = .0049); however, this did not cross the statistical significance threshold of P = .0044 as prespecified in the study protocol, and was therefore considered statistically insignificant.
Similarly, there was no significant difference in the secondary end points of ORR (P = .7276 vs P = .4085) and CBR (P = .9573 vs P = .6382) in the entire study population or the hormone receptor-negative subgroup, respectively.
Dr Hurvitz reported increased rates of any-grade stomatitis (67% vs 32%), diarrhea (57% vs 47%), neutropenia (38% vs 25%), and anemia (31% vs 16%) in patients who received everolimus compared with the placebo group. Additionally, on-treatment deaths due to an adverse event (AE) occurred in 3.6% of patients in the everolimus arm, compared with 0 in the placebo arm. All except 1 of these deaths occurred within 15 months of the beginning of the study, she said, adding that this may be associated with a lack of experience in managing the AEs of everolimus in combination with chemotherapy. Careful monitoring and early management of AEs in patients who receive everolimus and chemotherapy is therefore important, she concluded.
- © 2014 SAGE Publications