Summary
More cancer patient-specific data are needed to guide prevention and treatment of venous thromboembolism in patients with cancer. Longer-term treatment decision factors include bleeding risk and sex. Catheter removal in the event of thrombosis should be considered carefully. Direct oral anticoagulants are discouraged.
- pulmonary embolism
- venous thromboembolism
- catheter-related thrombosis
- cancer
- management
New Insights in Treatment
Stephan Moll, MD, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA, reviewed the decision-making process in treating patients with pulmonary embolism (PE) or venous thromboembolism (VTE).
Treatment at the time of PE or VTE diagnosis is guided by how sick the patient is. Outpatient treatment is feasible for half of patients with PE and for those with deep vein thrombosis (DVT) who are mobile at the time of clinical assessment. Risk assessment scoring tools to assess PE-related 30-day mortality [Barra SNC et al. Clin Cardiol. 2013] include the Hestia criteria [Zondag W et al. J Thromb Haemost. 2011], which can assess patients with PE suitable for outpatient management irrespective of right ventricular function [Zondag W et al. J Thromb Haemost. 2013].
Catheter-directed thrombolysis of newly diagnosed DVT seems to lessen postthrombotic syndrome at the expense of increased risk of bleeding [Enden T et al. Lancet. 2012]. The ATTRACT trial [Vedantham S et al. Am Heart J. 2013] is further assessing the value of catheter-directed thrombolysis.
At the time of diagnosis, the risk of mortality associated with PE can be assessed based on blood pressure, serum cardiac enzymes (troponin or brain natriuretic peptide levels), and right ventricle function [Meyer G et al. N Engl J Med. 2014; Jaff MR et al. Circulation. 2011; Torbicki A et al. Eur Heart J. 2008]. Concerning PE, thrombolytic therapy is indicated only for high-risk patients, although the risks and benefits of thrombolytic therapy in younger patients with submassive PE (i.e., right heart strain plus cardiac enzyme positivity) remain unclear.
Another treatment decision at diagnosis is whether to use anticoagulation with low-molecular-weight heparin (LMWH) and warfarin or one of the non–vitamin K antagonist oral anticoagulants (NOACs). NOACs are a good treatment choice for inpatient/outpatient treatment of patients with mild to moderate VTE, and they should be discussed for patients receiving long-term warfarin. NOACs are not recommended for patients with renal impairment, appreciable liver disease, or heightened risk of bleeding, or for very underweight or severely obese patients. Low-molecular-weight heparin is still the gold standard in patients with cancer-associated DVT or PE.
In the early days following diagnosis, compression stockings had no benefit in preventing postthrombotic syndrome in a placebo-controlled study [Kahn SR et al. Lancet. 2014].
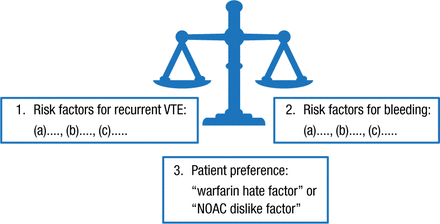
The length of anticoagulant treatment (ie, 3 months vs long term) is weighed on an individual basis (Figure 1).
Longer-Term Anticoagulant Treatment
NOAC, non–vitamin K antagonist oral anticoagulant; VTE, venous thromboembolism.
Reproduced with permission from S Moll, MD.
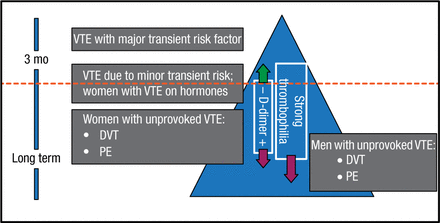
In the longer term, length of anticoagulation decision factors include whether a VTE was provoked by a major risk factor or was unprovoked (Figure 2), and, in the intermediate-risk-of-recurrence group (ie, patients with VTE associated with minor risk factors, eg, minor surgery, minor immobility, and estrogen therapy), D-dimer and thrombophilia testing. A negative D-dimer (on and/or off anticoagulation) predicts a lower risk of recurrence and pushes the patient up in the “recurrence triangle”; a positive D-dimer and finding of a strong thrombophilia push the patient down in the triangle, indicating a higher risk of recurrence.
Risk-of-Recurrence Triangle: How Long to Anticoagulate?
DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Reproduced with permission from S Moll, MD.
Finally, patient education is important to optimize outcomes and patient satisfaction. The Clot Connect information resource (www.clotconnect.org) is a nonprofit educational program of the University of North Carolina for patients with DVT or PE and health care professionals looking after them.
Keeping the Pipes Open in Cancer
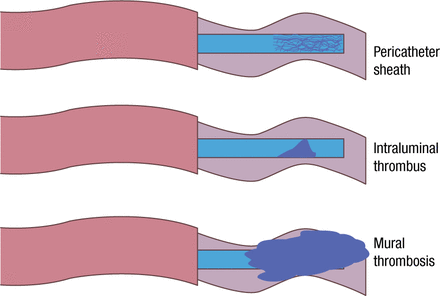
William Geerts, MD, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada, spoke on the management of catheter-related thrombosis (CRT) in patients with cancer. Central venous catheters (CVCs; peripherally inserted central catheters, midline catheters, tunneled and nontunneled central catheters, and implanted ports) are used in > 25% of inpatients and more than half of those receiving intensive care. Increasingly, the approach is used for outpatients. Resulting CRT includes formation of a pericatheter sheath of fibrin, intraluminal thrombosis, and superficial or deep venous thrombosis (Figure 3) [Baskin JL et al. Lancet. 2009].
Types of Thrombosis
Reproduced with permission from W Geerts, MD.
Adapted from Baskin JL et al. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet. 2009;374:159–169.
The resulting pathophysiology can involve local venous injury at the insertion site, venous injury and diminished blood flow due to fibrin deposition, and mural thrombosis. CRT is uncomfortable and anxiety producing; necessitates catheter removal and replacement, which increase the risk of infection; can require anticoagulant treatment with its associated risks; and increases health care costs. CVCs are associated with an approximately 7-fold increase in the risk of upper extremity vein thrombosis [Joffe HV et al. Circulation. 2004], which are asymptomatic in up to 60% of cases.
Catheter-related risk factors of CRT include the type of CVC, catheter size and composition, number of lumens, catheter insertion site and technique, and length of use. Patient- or therapy-related risk factors include the type and extent of cancer, chemotherapy, prior CRT or CVC, and infection. Peripherally inserted central catheters are associated with a greater risk of CRT [Chopra V et al. Lancet. 2013], especially with increasing catheter size [Evans RS et al. Chest. 2010], and major complications in patients with cancer.
When CRT is present, a catheter should not be removed just because of thrombosis; indications for removal are failure to function, suspected infection, or because it is not needed [Debourdeau P et al. J Thromb Haemost. 2013; Kovacs MJ et al. J Thromb Haemost. 2007]. Treatment can involve intermediate- or full-dose LMWH, full-dose LMWH, and direct oral Xa or IIa inhibitor. CRT prevention is not achieved using flushes with heparinized saline or warfarin. Instead, avoidance of catheter use if possible, use of the smallest catheter, catheter insertion by experts using ultrasound guidance, and anticoagulant prophylaxis are prudent in patients with cancer.
Prevention and Treatment of Cancer-associated Thrombosis (CAT)
Agnes Lee, MD, MSc, University of British Columbia, Vancouver, British Columbia, Canada, discussed strategies for the prevention and treatment of VTE in patients with cancer.
Inpatient anticoagulant prophylaxis is prudent, if there are no contraindications [Lyman et al. J Clin Oncol. 2013; Kahn SR et al. Chest. 2012; Mandala M et al. Ann Oncol. 2011], for several reasons: the risk of thrombosis is high for patients with various malignancies, most are admitted to hospital for another acute condition like infection, mobility is likely to be reduced, and VTE is associated with significant morbidity and mortality. Of note, although VTE risk is reduced by anticoagulant therapy, up to one-third of hospitalized patients have contraindications for prophylactic anticoagulation [Zwicker JI et al. J Clin Oncol. 2014]. Patients with cancer who are in one or more of the following categories likely will benefit from prophylaxis: aged ≥ 70 years, body mass index ≥ 30 kg/m2, reduced mobility, prior VTE, an acute medical condition, and current hormone therapy [Kahn SR et al. Chest. 2012].
In acutely ill patients in general, parenteral anticoagulants (eg, enoxaparin, dalteparin, and fondaparinux) can reduce the risk of VTE during hospitalization [Cohen AT et al. BMJ. 2006; Leizorovicz A et al. Circulation. 2004; Samama MM et al. N Engl J Med. 1999]. Prophylaxis with direct oral anticoagulants apixaban or rivaroxaban is noninferior to LMWH, but it increases bleeding [Cohen AT et al. N Engl J Med. 2013; Goldhaber SZ et al. N Engl J Med. 2011], with no information yet available specifically for patients with cancer. Available data support the use of LMWH, but randomized controlled trials specifically involving patients with cancer are needed to better define the risk–benefit balance.
Regarding extended prophylaxis following hospital discharge, clinical trials have not yet indicated the overall benefit of the strategy, due to the risk of increased bleeding (Table 1) [Cohen AT et al. N Engl J Med. 2013; Goldhaber SZ et al. N Engl J Med. 2011; Hull RD et al. Ann Intern Med. 2010].
Extended Prophylaxis Increases Major Bleeding.
Apixaban, rivaroxaban, edoxaban, and dabigatran have proven noninferior to treatment with vitamin K antagonists in the prevention of VTE [Agnelli G et al. N Engl J Med. 2013; Hokusai-VTE Investigators. N Engl J Med. 2013; Schulman S et al. N Engl J Med. 2009; EINSTEIN DVT and PE. N Engl J Med. 2010 and 2012]. While data suggest that these direct oral anticoagulants provide a benefit similar to that of conventional treatment in highly selected patients with cancer [Vedovati MC et al. Chest. 2014], their use is discouraged in this population. Drug clearance can be affected by renal dysfunction and hepatic metastases, and drug–drug interactions can diminish treatment efficacy and increase the risk of bleeding, and these drugs have not been compared with LMWH, the standard-of-care therapy for cancer-associated thrombosis.
- © 2014 SAGE Publications