Summary
Echocardiography and other multimodality imaging tools are invaluable for assessing cardiac structure and function. This technology is being used to assess cardiac mechanics in pericardial diseases, distinguish constrictive pericarditis from restrictive cardiomyopathy, and identify ischemic cardiomyopathy patients. Besides having prognostic value, these tools are useful in the management and treatment of patients with cardiovascular disease.
- transthoracic echocardiography
- cardiac structure
- heart failure
- ejection fraction
- pericardial disease
- multimodality imaging
- pericarditis
- cardiomyopathy
- ischemic heart disease
- STICH
- NCT00023595
Transthoracic echocardiography is now routinely used to evaluate cardiac structure and function; to measure left ventricular ejection fraction (LVEF); to diagnose, manage, and treat heart failure (HF); and to obtain prognostic information in patients with cardiovascular (CV) disease (Class I; Level of Evidence C) [McMurray JJV et al. Eur Heart J. 2012]. E. Donal, MD, Hôpital Pontchaillou, Rennes, France, discussed how to interpret different parameters detected by imaging technology in patients with heart failure with preserved ejection fraction (HFpEF).
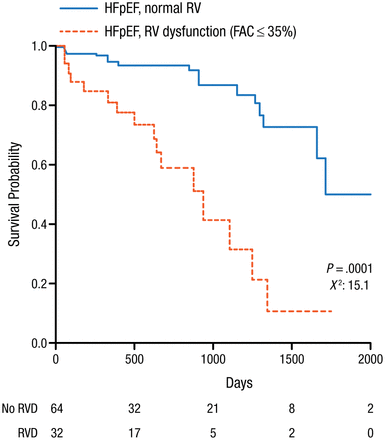
HFpEF is a major and growing worldwide health problem that is diagnosed in part with the use of echocardiography. Echocardiography can detect signs of vascular stiffness, cardiac dysfunction, and atrial fibrillation (AF). Signs consistent with HFpEF include LVEF > 50%, left ventricular (LV) filling dysfunction, and increases in left atrial (LA) volume index and LV mass index. LA strain and LA stiffness are the most accurate indices in identifying patients with HFpEF [Kurt M et al. Circ Cardiovasc Imaging. 2009]. Impaired LA function may be a marker of severity of HFpEF and is associated with a higher prevalence of prior HF hospitalization and history of AF, as well as worse LV systolic function [Santos AB et al. Eur J Heart Fail. 2014]. Pulmonary hypertension (PH) is present in 83% of patients with HFpEF and the presence of PH can distinguish HFpEF from preclinical hypertensive heart disease and is a strong predictor of mortality [Lam CS et al. J Am Coll Cardiol. 2009]. Right ventricular (RV) dysfunction (RV fractional area change < 35%) is present in 33% of HFpEF patients and is associated with greater severity, comorbidity, and mortality (Figure 1) [Melenovsky V et al. Eur Heart J. 2014].
RVD in HFpEF Patients Associated With Higher Mortality
FAC, fractional area change; HFpEF, heart failure with preserved ejection fraction; RV, right ventricule; RVD, right ventricular dysfunction.
Reprinted from Melenovsky V et al. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014; Epub ahead of print 29 May 2014. doi:10.1093/eurheartj/ehu193. Accessed 12/19/2014. By permission of European Society of Cardiology.
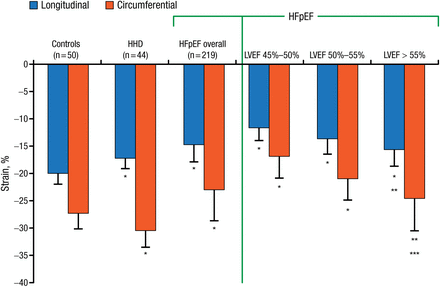
Reduced LV compliance, RV remodeling, and low tricuspid annular plane systolic excursion are also significant pathophysiologic predictors of adverse outcomes in HFpEF patients [Burke MA et al. Circ Heart Fail. 2014]. Echocardiography is useful for either the diagnosis or exclusion of suspected HF (systolic and diastolic) with the measurement of LV longitudinal systolic function [Vinereanu D et al. Eur J Heart Fail. 2005]. Depressed longitudinal and radial deformation indicates diastolic HF [Wang J et al. Eur Heart J. 2008]. Longitudinal and circumferential strain detected by strain imaging is significantly (P < .0001) lower in HFpEF patients (Figure 2) [Kraigher-Krainer E et al. J Am Coll Cardiol. 2014].
Longitudinal and Circumferential Strain Lower in HFpEF Patients
HFpEF, heart failure with preserved ejection fraction; HHD, hypertensive heart disease; LVEF, left ventricular ejection fraction.
*P < .0001 compared with controls and between HHD and HFpEF overall for longitudinal strain and circumferential strain. **LVEF-adjusted P < .001 compared with controls. ***P = .0002 compared with controls.
Adapted from Kraigher-Krainer E et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447-456.
HFpEF can be difficult to identify, but exercise echocardiography can play an important role in diagnosis. During exercise, HFpEF patients display lower peak VO2 coupled with significantly blunted increases in heart rate, stroke volume, LVEF, and LV end-systolic volume compared with controls [Abudiab MM et al. Eur J Heart Fail. 2013].
Allan L. Klein, MD, Cleveland Clinic, Cleveland, Ohio, USA, discussed the use of multimodality imaging to assess cardiac mechanics in pericardial diseases and as well as the use of imaging to distinguish constrictive pericarditis from restrictive cardiomyopathy. Using a case example of a man, aged 47 years, with worsening exertional dyspnea and cough, findings from echocardiography and cardiac magnetic resonance imaging (CMR) revealed organizing pericardial effusion, inferior vena cava plethora, abnormal septal bounce, respiratory Doppler variation, interventricular respirophasic shift, increased pericardial thickness, and the presence of late gadolinium enhancement of pericardium. Dr Klein referred this patient for pericardiectomy and prescribed anti-inflammatory medication until the time of surgery.
Surgery revealed constrictive pericarditis, which is very often hard to distinguish from restrictive cardiomyopathy. Tissue Doppler echocardiography has been used successfully to distinguish between these 2 diseases [Rajagopalan N et al. Am J Cardiol. 2001]. In particular, the ratio between lateral and septal mitral annular velocity is significantly reduced in constrictive pericarditis compared with controls and restrictive cardiomyopathy patients [Choi JH et al. JACC Cardiovasc Imaging. 2011]; moreover, reduced lateral annular velocity is correlated with pericardial thickness.
Constrictive pericarditis is defined by epicardial tethering and pericardial constraint in fibrotic or inflamed pericardium with LV filling limited to a circumferential direction. Intrinsic myocardial disease with subendocardial dysfunction and LV filling limited to a longitudinal direction defines restrictive cardiomyopathy [Sengupta PP et al. JACC Cardiovasc Imaging. 2008]. CMR shows depressed LV anterolateral wall strain and RV free wall longitudinal systolic strain but preserved LV septal wall systolic strain in constrictive pericarditis patients, which is improved by pericardiectomy [Kusunose K et al. Circ Cardiovasc Imaging. 2013]. There is also a significant inverse correlation between pericardial thickness and respective ventricular strains not seen in restrictive cardiomyopathy patients (strain reverses).
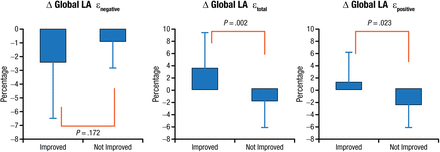
An echocardiography evaluation of LA mechanics in constrictive pericarditis showed impaired mechanics, presumably because of the constrictive tethering process involving the left atrium. Functional improvement in global LA strain parameters were noted following pericardiectomy (Figure 3) [Motoki H et al. J Am Soc Echocardiogr. 2013].
Functional Improvement Before and After Pericardiectomy
Global LA longitudinal strain (ε) included peak negative strain (εnegative), peak positive strain (εpositive), and the sum of those values, total LA strain (εtotal).
LA, left atrial.
Adapted from J Am Soc Echocardiogr. Vol 26, Motoki H et al. Changes in left atrial mechanics following pericardiectomy for pericardial constriction, 640-648. Copyright (2013), with permission from American Society of Echocardiography.
Dr Klein concluded that constrictive pericarditis is characterized by perimyocardial tethering involving both the ventricles and atria, decreased global circumferential strain, and preserved longitudinal strain. Regional longitudinal LV and LA strain is impaired in constrictive pericarditis and improves after pericardiectomy as well as with medical therapy.
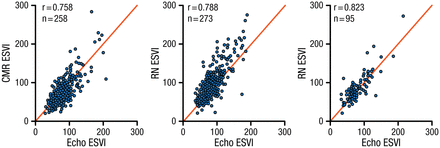
Jae K. Oh, MD, Heart Vascular Stroke Institute, Samsung MC, Korea, focused his presentation on the diagnosis, treatment, and prognosis of dilated cardiomyopathy (DCM). As cardiomyopathy progresses, the LV size increases, ejection fraction (EF) decreases, and mitral regurgitation increases. Echocardiography Core Laboratory analysis of baseline echocardiographic findings from the Comparison of Surgical and Medical Treatment for Congestive Heart Failure and Coronary Artery Disease (STICH) study [NCT00023595] in ischemic cardiomyopathy patients demonstrated a wide spectrum of LV shape, function, and hemodynamic impairment [Oh JK et al. J Am Soc Echocardiogr. 2012]. The addition of coronary artery bypass grafting (CABG) to medical therapy reduced mortality, sudden death, and fatal pump failure events. The addition of surgical ventricular reconstruction (SVR) to CABG resulted in no overall benefit, although a subgroup analysis suggested that patients with less dilated LV and better LVEF function benefit more from SVR [Oh JK et al. Eur Heart J. 2013]. An examination of methods for measuring LV end-systolic volume index (CMR, radionuclide imaging, and echocardiography) shows good correlation among the imaging modalities (Figure 4).
Echo vs CMR vs RN
CMR, cardiac magnetic resonance imaging; Echo, echocardiography; ESVI, end-systolic volume index; RN, radionuclide imaging.
Reprinted from Oh JK et al. Influence of baseline left ventricular function on the clinical outcome of surgical ventricular reconstruction in patients with ischaemic cardiomyopathy. Eur Heart J. 2013;34:39-47. By permission of European Society of Cardiology.
There was no significant difference between medical therapy alone and medical therapy plus CABG with respect to the primary end point of death from any cause, but patients receiving only CABG had lower rates of death from CV causes and of death from any cause or hospitalization for CV causes compared with medical therapy alone [Velazquez EJ et al. N Engl J Med. 2011].
The presence of 3-vessel coronary artery disease, EF below the median (27%), and end-systolic volume index above the median (79 mL/m2) influenced these findings [Panza JA et al. J Am Coll Cardiol. 2014]. After ≥ 2 years, patients with 2 to 3 prognostic factors had reduced mortality with CABG compared with those who only received medical therapy. SVR added to CABG reduced LV volume but did not decrease the rate of death or hospitalization for cardiac causes, compared with CABG alone [Jones RH et al. N Engl J Med. 2009].
Two novel imaging tools are global longitudinal strain, (GLS) CMR, cardiac magnetic resonance imaging; often used to measure LV longitudinal deformation, and speckle tracking echocardiography. GLS is a good prognostic tool for predicting major adverse cardiac events and is superior to LVEF [Kalam K et al. Heart. 2014]. Speckle tracking echocardiography is a sensitive method for assessing ventricular function and may detect myocardial dysfunction in sepsis cases not seen with conventional echocardiography [Orde SR et al. Crit Care. 2014]. Prof Oh concluded that echocardiography is a reliable tool for detecting DCM, as well as useful for guiding treatment.
Myocardial function assessment in ischemic heart disease depends on a good understanding of cardiac mechanics. The complex interplay between the tissue structure/shape, force development, cavity pressure development, ejection by wall deformation, and the potential to adapt to changing circumstances is of great importance when diagnosing CV abnormalities. B. Bijnens, MD, Institució Catalana de Recerca i Estudis Avançats, Barcelona, Spain, reviewed the mechanics and physiology of ischemic heart disease, and discussed important points to consider when using this information for the assessment of ventricular function.
As an example, he noted that strain rate imaging indices (myocardial strain and strain rate) are useful in detecting the magnitude and rate of myocardial deformation in asymptomatic patients with severe mitral regurgitation. Estimation of myocardial shortening and thickening reflect the radial mechanics of the heart and provide a sensitive means for detecting regional myocardial dysfunction, including ischemia. Formulas have been developed to identify patients at risk of myocardial damage based on passive load, tissue elasticity, and regional deformation.
Acute ischemia causes systolic deformation, which leads to postsystolic thickening. Increased afterload significantly worsens regional systolic and diastolic dysfunction, while progression of regional ischemia results in the loss of relaxation.
Ischemia followed by reperfusion results in myocardial edema (myocyte swelling and myofibrillar edema) and stunning, or contractile abnormality due to changes in force development [Bragadeesh T et al. Heart. 2007]. The identification of an acute increase in regional wall thickness in a reperfused infarct zone by cardiac ultrasound can be useful for monitoring the presence, extent, and resolution of the edema associated with reperfusion injury [Turschner O et al. Eur Heart J. 2004].
Prof Bijnens concluded that deformation is linked to cardiac force, perfusion, and flow/functional reserves. When there is a difference in force development or deformation in neighboring segments, postsystolic deformation increases. Systolic deformation decreases with decreasing flow and increased transmural fibrosis and myocardial stiffness.
- © 2014 SAGE Publications