Summary
The age-adjusted incidence of a new diagnosis of heart failure (HF) has not changed over the past decades and, in some patient populations, is actually decreasing. However, because our society is aging and mortality from HF has been reduced, the prevalence of people living with a diagnosis of HF has increased and is currently fueling a cycle of hospitalization for HF. This article discusses the impact of HF drugs in patients with diabetes, the impact of new glucose-lowering drugs on HF, sodium glucose cotransporter 2 (SGLT-2) inhibitors.
- diabetes mellitus
- heart failure
The age-adjusted incidence of a new diagnosis of heart failure (HF) has not changed over the past decades and, in some patient populations, is actually decreasing. However, because our society is aging and mortality from HF has been reduced, the prevalence of people living with a diagnosis of HF has increased and is currently fueling a cycle of hospitalization for HF, stated Veronique L. Roger, MD, MPH, Mayo Clinic, Rochester, Minnesota, USA. There has been an increase in the proportion of HF patients with preserved ejection fraction (HFpEF ≥ 50%) when compared to reduced ejection fraction (HFrEF < 50%). At the Mayo Clinic, the proportion of HF patients diagnosed with HFpEF increased from 38% in the 1986-to-1990 period to 54% in the 1998-to-2001 period [Owan TE et al. N Engl J Med. 2006]. In Olmsted County, Minnesota, the proportion of HFpEF patients is estimated to be 55% [Bursi et al. JAMA. 2006], with 53% of new cases categorized as HFpEF over the last decade [Gerber Y et al. AHA 2014 (abstr 15685)].
The increase in diabetes mellitus (DM) is associated with the increasing prevalence of HFpEF. The population-attributable risk for HF associated with DM was 9% in 2000 [Dunlay SM et al. Am J Med. 2009], based on a 2.65-fold increased risk for developing HF with diabetes. As the prevalence of DM increases, the population-attributable risk is expected to increase, especially as other causes of HF (eg, coronary artery disease) are becoming less impactful.
An effect of diabetes on HF may be mediated by subclinical myocardial injury and non-atherosclerotic mechanisms. For instance, studies have shown a positive-graded relationship between categories of diabetes (no diabetes, prediabetes, and diabetes) and detectable levels of high-sensitivity troponin elevation [Selvin E et al. Circulation. 2014]. Categories of diabetes were positively associated with the risk of HF and all-cause mortality, in those with and without troponin elevation. The association of elevated troponins with HF was stronger than that for coronary events, leading the authors to suggest that the risk of HF is unlikely to be mediated by myocardial infarction or microvascular disease.
IMPACT OF HF DRUGS IN PATIENTS WITH DIABETES
In patients with HFrEF, with or without DM, there is a similar relative risk reduction in morbidity and mortality with all the evidence-based, guideline-recommended pharmacologic and device therapies, according to data reviewed by John J. V. McMurray, University of Glasgow, Glasgow, Scotland, United Kingdom. However, in patients with DM, similar relative risk reductions translate to greater absolute risk reductions because of their higher absolute risk. Although DM itself is associated with more adverse events, available evidence-based therapies for HFrEF are safe and well tolerated in patients with DM, he stated.
The SOLVD-Treatment trial in the 1990s found that the angiotensin-converting enzyme inhibitor enalapril was equally effective regardless of DM status in reducing cardiovascular (CV) death or hospitalization for HF (P Interaction = .54). Regarding β-blockers, HF and CV hospitalization was reduced with metoprolol in MERIT-HF [Deedwania PC et al. Am Heart J. 2005], and all-cause mortality was reduced with carvedilol in COPERNICUS [Mohacsi P et al. Circulation. 2001]. A similar reduction in mortality in patients with or without diabetes was found in an analysis of the β-blocker trials [Deedwania PC et al. Am Heart J. 2005].
β-Blockers are safe and well tolerated in patients with HF and DM, although there are misperceptions regarding their safety, leading to suboptimal use. Data from MERIT-HF showed that study drug discontinuation was similar for placebo and metoprolol regardless of DM status. Hypoglycemia was rare with metoprolol and placebo (0.8% and 0.6%, respectively).
Mineralocorticoid receptor antagonists are also underused in patients with DM and HFrEF, stated Prof McMurray. In patients with severe HFrEF, there was a similar reduction in CV death or HF hospitalization with spironolactone in those with DM (HR, 0.68; 95% CI, 0.52 to 0.90) and without DM (HR, 0.64; 95% CI, 0.54 to 0.75; P Interaction =.67) in the RALES study. Eplerenone had a trend toward a greater effect on CV death or HF hospitalization in patients with DM (HR, 0.54; 95% CI, 0.42 to 0.70) than without DM (HR, 0.71; 95% CI, 0.58 to 0.88; P Interaction = .09) in the EMPHASIS-HF study [Zannad F et al. Heart Failure. 2012]. Mineralocorticoid receptor antagonists are also safe and well tolerated in this patient group, with a low rate of hyperkalemia (K+ > 6.0 mmoL/L) in all patients taking eplerenone and those with diabetes (2.5% vs 3.8%) [Pitt B et al. ESC 2011] and with a similar change in estimated glomerular filtration rate (−3.2 and −4.9 mL/min/1.73 m2, respectively).
Unpublished data show that the reduction in CV death or HF hospitalization was similar in patients with or without DM with digoxin in the DIG trial (HR, 0.90 vs 0.83; P Interaction = .27) and the novel angiotensin receptor blocker/neprilysin inhibitor LCZ696 (HR, 0.84 vs 0.77; P Interaction = .40) in the PARADIGM-HF study. Ivabradine was favorable regardless of diabetes history in the SHIFT study (HR, 0.81 vs 0.83; P Interaction = .86) [Swedberg K et al. Lancet. 2010]. In the A-HeFT trial [Taylor AL et al. N Engl J Med. 2004], unpublished subgroup analysis showed that the improvement in survival with hydralazine plus isosorbide dinitrate was similar in African-American patients regardless of diabetes status.
In SCD-HeFT—the only large trial of an implantable cardioverter defibrillator in HF—a subgroup analysis suggested that patients with DM did not receive the same benefit as those without DM (HR, 0.95 vs 0.67) [Bardy GH et al. N Engl J Med. 2005]. However, there was no suggestion of this in the MADIT II study of postinfarct patients with a low ejection fraction, suggesting a false-positive finding, stated Prof McMurray. Cardiac resynchronization therapy was beneficial regardless of DM status in reducing death or CV hospitalization and death or HF hospitalization in the CARE-HF trial, with a similar ∼ 7% improvement in left ventricular ejection fraction and NYHA class (Table 1) [Hoppe UC et al. Diabetes Care. 2007].
Improvements With Cardiac Resynchronization Therapy in Patients With and Without Diabetes
IMPACT OF NEW GLUCOSE-LOWERING DRUGS ON HF
No glucose-lowering drug or regimen has been shown to improve HF outcomes in patients with DM, stated Benjamin M. Scirica, MD, MPH, Brigham and Women's Hospital, Boston, Massachusetts, USA. Notably, signals of HF exacerbation and increased hospitalization for HF have been seen in randomized clinical trials of thiazolidinediones (TZD), dual peroxisome proliferator-activated receptor (PPAR) agonists with alpha-gamma activity, and dipeptidyl peptidase 4 (DPP-4) inhibitors.
One meta-analysis showed an increased risk of HF with TZDs vs standard glucose management [Castagno D et al. Am Heart J. 2011]. Data from the PROACTIVE study not only demonstrated a higher HF rate but also found an increased rate of edema with pioglitazone vs placebo (27.4% vs 15.9%; P < .001) [Erdmann E et al. Diabetes Care. 2007]. Edema from PPAR agonists is thought to be due to fluid retention, decreased glycosuria, and increased adiposity. However, whether this is a nuisance side effect that can be managed or a bad prognostic marker of worsening HF is unclear, stated Dr Scirica. A signal of an increase in incident HF with pioglitazone and rosiglitazone (greater with the latter) was seen in another meta-analysis [Lago RM et al. Lancet. 2007].
Similarly, increased hospitalization for HF (HR, 1.22; 95% CI, 0.94 to 1.59; P = .14) and risk for HF-related serious adverse events (HR, 1.24; 95% CI, 0.99 to 1.66; P = .06) and edema (P < .001) have been found with the dual PPAR agonist aleglitazar versus placebo, although with no ischemic effects, in the AleCardio trial [Lincoff AM et al. JAMA. 2014]. As with TZDs, for dual PPAR agonists, it remains unclear to what extent these signals translate into absolute rate increases in HF risk.
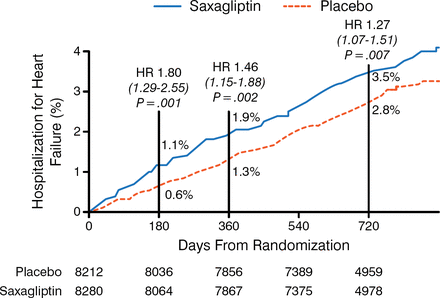
The DPP-4 inhibitors saxagliptin and alogliptin, when compared with placebo, had no effect on the primary CV end point in the SAVOR and EXAMINE trials, respectively [Scirica BM et al. N Engl J Med. 2013; White WB et al. N Engl J Med. 2013]. In SAVOR, there was an absolute increased risk of hospitalization for HF with saxagliptin vs placebo (3.5% vs 2.8%; HR, 1.27; 95% CI, 1.07 to 1.51; P = .007); however, no increase in peripheral edema was found. Although not enough end points were available to achieve statistical significance, EXAMINE suggested a similar relative increase. SAVOR found that the HF risk was higher early, with fairly similar rates after 12 months in both arms (Figure 1) [Scirica BM et al. Circulation. 2014]. More evidence from other trials is needed to determine whether this HF signal was a chance observation, whether the effect is specific to DPP-4 inhibitors or occurs with all incretin drugs, or whether it is a general effect of all glucose-lowering drugs that can exacerbate the disease process in patients at high risk of HF.
Time to Hospitalization for Heart Failure in the SAVOR Trial
CVD/HF post hoc composite: 5.9% vs 5.0%; HR, 1.15; P = .039. Black vertical lines indicate landmark analysis at 12 months: 1.7% vs 1.5%; HR, 1.09; P = .51; time-varying interaction, P = .017.
Adapted from Scirica BM et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–1588. With permission from American Heart Association, Inc.
SGLT-2 INHIBITORS
The sodium glucose cotransporter 2 (SGLT-2) inhibitors have effects on sodium metabolism, fluid volume, and blood pressure (BP) that have the potential to be beneficial in regard to HF, stated Bruce Neal, MD, The George Institute for Global Health, Sydney, Australia. Although no data on HF outcomes have been reported, ongoing large-scale trials with SGLT-2 inhibitors should provide clear evidence of the effect on HF.
A systematic review of studies of SGLT-2 inhibitors in patients with type 2 DM (45 studies vs placebo; 13 studies vs active comparator) showed a mean reduction in HbA1c of 0.79%, systolic BP of 3.8 mm Hg, diastolic BP of 1.8 mm Hg, and body weight of 1.74 kg [Vasilakou D et al. Ann Intern Med. 2013]. Prof Neal noted that there was a 30% to 40% reduced risk of albuminuria found with SGLT-2 inhibition in patients with type 2 DM and chronic kidney disease in a randomized trial [Yale JF et al. Diabetes Obes Metab. 2013].
- © 2014 MD Conference Express®