Summary
This article reports on the primary outcomes of the prospective, randomized, single-blind, multicenter EVOLVE II Clinical Trial to Assess the SYNERGY Stent System for the Treatment of Atherosclerotic Lesion(s) [EVOLVE II; NCT01665053], which assessed the safety and effectiveness of 2 different everolimus-eluting stents, the second-generation SYNERGY stent and the PROMUS Element Plus durable-polymer drug-eluting stent.
- cardiology clinical trials
- interventional techniques & devices coronary artery disease
Dean J. Kereiakes, MD, Christ Hospital Heart and Vascular Center, Cincinnati, Ohio, USA, reported on the primary outcomes of the prospective, randomized, single-blind, multicenter EVOLVE II Clinical Trial to Assess the SYNERGY Stent System for the Treatment of Atherosclerotic Lesion(s) [EVOLVE II; NCT01665053], which assessed the safety and effectiveness of 2 different everolimus-eluting stents, the second-generation SYNERGY stent and the PROMUS Element Plus durable-polymer drug-eluting stent (DP-DES).
The polymer in a DES acts as a drug reservoir and allows for the programmed release of the drug. Once drug release is complete, the polymer has no function and, if it becomes physically damaged, may be detrimental, leading to late/very late stent thrombosis (ST), chronic inflammation, late restenosis, or hypersensitivity. The SYNERGY stent features a platinum chromium platform coated with poly (lactic-co-glycolic acid). Everolimus is subsequently applied to the surface of the biodegradable coating.
Drug release occurs fairly constantly and in parallel with polymer degradation. The potential value of the design has been indicated in a porcine model and in the 30-day EVOLVE trial involving 291 patients [Meredith IT et al. J Am Coll Cardiol. 2012]. These data prompted the EVOLVE II trial, in which 1684 patients were randomized to treatment with the DP-DES (n = 842) or the SYNERGY stent (n = 842). Exclusion criteria were left main disease, chronic total occlusion, saphenous vein graft, in-stent restenosis, and recent STEMI.
Patients received acetylsalicylic acid plus clopidogrel, ticlopidine, prasugrel, or ticagrelor for at least 6 months. The primary end point in the intention-to-treat and per-protocol populations was target lesion failure at 12 months (defined as a composite of cardiac death, myocardial infarction [MI], or ischemia-driven revascularization). Secondary end points included the individual components of target lesion failure, definite or probable ST (Academic Research Consortium definition), technical and clinical success of stenting, and longitudinal deformation of the stent.
Baseline characteristics in the 2 groups were similar for a number of demographic and clinical features. Procedural and postprocedural characteristics and outcomes were also comparable between groups. Antiplatelet medication use was similar at 6 and 12 months.
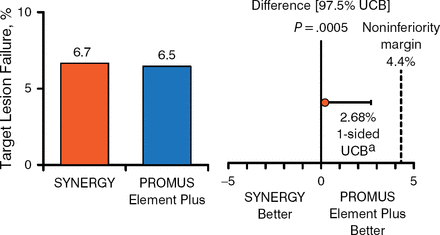
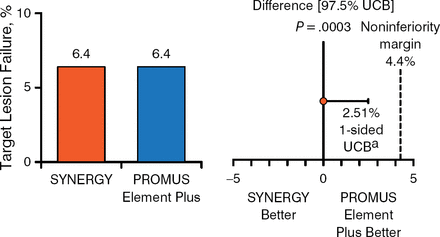
At 1 year, complete follow-up was available in 806 (96.2%) patients in the DP-DES group and 831 (98.2%) patients in the SYNERGY group. The primary end point in the intention-to-treat and per-protocol populations respectively occurred in 6.7% and 6.5% of patients receiving the SYNERGY or DP-DES, respectively, and 6.4% and 6.4% of those receiving the SYNERGY or DP-DES, respectively. Thus, noninferiority was proven to a high level of significance between the 2 stents (Figures 1 and 2).
Primary End Point in the Intention-to-Treat Population
aOne-sided 97.5% Farrington-Manning upper confidence bound.
UCB, upper confidence bound.
Reproduced with permission from DJ Kereiakes, MD.
Primary End Point in the Per-Protocol Population
aOne-sided 97.5% Farrington-Manning upper confidence bound.
UCB, upper confidence bound.
Reproduced with permission from DJ Kereiakes, MD.
In addition, 12-month rates of revascularization, stent-related thrombosis, cardiac death, target vessel-related MI, and clinically indicated target lesion revascularization were similar between stents. Two definite and 3 probable cases of ST occurred with the DP-DES. The SYNERGY stent was associated with 2 definite cases and 1 probable case of ST.
The data demonstrate the noninferiority of the SYNERGY stent compared with the PROMUS Element Plus DP-DES for target lesion failure at 1 year. Longer-term efficacy and safety analyses are currently ongoing.
- © 2014 MD Conference Express®