Summary
Hypertrophic cardiomyopathy (HCM) has a prevalence of 1 in every 500 individuals, making it the most frequently inherited cardiomyopathy, and it results in the fibrosis and hypertrophy of the left ventricle. This article discusses data from the Inhibition of the Renin Angiotensin System With Losartan in Patients With Hypertrophic Cardiomyopathy [INHERIT; NCT01447654] trial, which investigated the use of losartan for HCM.
- inflammatory disease
- cardiology clinical trials
Losartan treatment in patients with hypertrophic cardiomyopathy (HCM) did not alter left ventricular mass from baseline over 12 months compared with placebo. Anna Axelsson, MD, The Heart Center, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark, presented data from the Inhibition of the Renin Angiotensin System With Losartan in Patients With Hypertrophic Cardiomyopathy [INHERIT; NCT01447654] trial.
HCM has a prevalence of 1 in every 500 individuals, making it the most frequently inherited cardiomyopathy, and it results in the fibrosis and hypertrophy of the left ventricle [Green JJ et al. JACC Cardiovasc Imaging. 2012]. Data from in vivo studies performed in animal models and humans indicate that angiotensin receptor blockers (ARBs) may have a benefit on diastolic function, left ventricular mass, exercise capacity, and myocardial fibrosis [Shimada YJ et al. JACC Heart Fail. 2013; Penicka M et al. J Mol Diagn. 2009; Araujo AQ et al. Am J Cardiol. 2005]. The purpose of the INHERIT trial was to evaluate the effect of the ARB losartan on morphology and function of the left ventricle in patients with HCM.
In the single-center, double-blind, phase 2 INHERIT trial, 133 adult patients with HCM were randomly assigned to receive losartan 100 mg/d or placebo for 12 months. All patients were in sinus rhythm upon inclusion into the study. Patients were excluded if they had a left ventricular ejection fraction < 50%, significant valvular disease, blood pressure > 140/90 mm Hg, an estimated glomerular filtration rate < 30 mL/min per 1.73 m2, were currently taking an ARB or angiotensin-converting enzyme inhibitor, or had septal reduction treatment within 6 months. At baseline, the mean age was 52 years and 36% of participants were women. In the study, 64%, 30%, and 6% of patients were classified as having NYHA functional class I, II, and II, respectively. In addition, 43% of patients were identified as having a disease-causing genetic mutation.
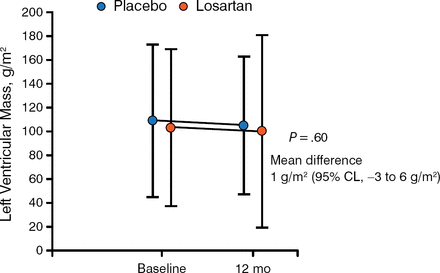
The primary end point was change in left ventricular mass as determined by magnetic resonance imaging or computed tomography imaging. Secondary end points included changes in left ventricular maximal wall thickness, outflow tract gradient, and fibrosis, as well as changes in diastolic function, exercise tolerance, and symptoms of HCM. In the study, 93% of patients were compliant with study medication as determined by pill count.
Effect of Losartan on Left Ventricular Mass in Patients With Hypertrophic Cardiomyopathy
95% CL, 95% confidence limit.
Reproduced with permission from A Axelsson, MD.
There was no significant difference in change in left ventricular mass from baseline among patients who received losartan or placebo at 12 months (P = .60). Similarly, there was no significant difference in change in maximal ventricular wall thickness, echocardiographic findings such as outflow gradient. In addition, a subgroup analysis demonstrated that there was no benefit with losartan treatment based on age, presence of a genetic mutation causing HCM, left ventricular outflow tract obstruction, maximal wall thickness, or history of myectomy or alcohol septal ablation.
The rate of adverse events (AEs) was similar among the losartan and placebo arms. AEs included sudden cardiac death, angioedema, hyperkalemia, renal impairment, worsening of NYHA functional class, and left ventricular outflow tract gradient. Seven patients discontinued therapy; unspecified symptoms led to discontinuation by 2 patients in the losartan arm and 1 patient in the placebo arm, 1 patient in the losartan arm discontinued treatment because of deterioration of renal function, angioedema caused 1 patient in the losartan arm to discontinue therapy, and 2 patients in the losartan arm were referred for septal reduction therapy and excluded from follow-up. In addition, 2 patients in the placebo arm died from sudden cardiac death.
In conclusion, Dr Axelsson stated that the results from the INHERIT trial indicate that losartan does not provide a benefit for left ventricular mass in patients with HCM; however, losartan treatment was safe and may be used, albeit with caution, for other indications in this population such as for treatment of hypertension.
- © 2014 MD Conference Express®