Summary
Statins have been demonstrated to reduce morbidity and mortality; however, adding other lipid-modifying therapies to statin treatment has not demonstrated a clear benefit. This article presents data from the Improved Reduction of Outcomes: Vytorin (Ezetimibe/Simvastatin) Efficacy International Trial [IMPROVE-IT; NCT00202878]. The purpose of the IMPROVE-IT trial was to evaluate the clinical benefit of combination therapy with ezetimibe plus simvastatin compared with simvastatin monotherapy in lowering LDL-C levels.
- cardiology clinical trials
- lipid disorders
Combination therapy with ezetimibe and simvastatin reduced the rate of cardiovascular (CV) death, myocardial infarction (MI), hospital admission for unstable angina (UA), coronary revascularization, and stroke in patients with acute coronary syndrome (ACS) compared with simvastatin alone. Christopher P. Cannon, MD, Brigham and Women's Hospital, Boston, Massachusetts, USA, presented data from the Improved Reduction of Outcomes: Vytorin (Ezetimibe/Simvastatin) Efficacy International Trial [IMPROVE-IT; NCT00202878].
Statins have been demonstrated to reduce morbidity and mortality; however, adding other lipid-modifying therapies to statin treatment has not demonstrated a clear benefit. Ezetimibe causes decreased cholesterol absorption by inhibiting the Niemann-Pic C1-like1 protein that is located primarily within the brush border of the epithelium of the gastrointestinal tract. The addition of a statin to ezetimibe therapy results in a synergistic decrease of approximately 20% in low-density lipoprotein cholesterol (LDL-C) reduction. The purpose of the IMPROVE-IT trial was to evaluate the clinical benefit of combination therapy with ezetimibe plus simvastatin compared with simvastatin monotherapy in lowering LDL-C levels The design and final baseline characteristics of the IMPROVE-IT trial were previously published [Blazing MA et al. Am Heart J. 2014; Cannon CP et al. Am Heart J. 2008].
In the multicenter, double-blind, phase 3 IMPROVE-IT trial, 18 144 patients with STEMI and NSTEMI, or UA who were aged ≥ 50 years were randomly assigned to receive simvastatin or ezetimibe plus simvastatin after conventional medical and interventional therapy [Blazing MA et al. Am Heart J. 2014]. For inclusion, patients were required to have an LDL-C level of 50 to 125 mg/dL (between 50 and 100 mg/dL if on lipid-lowering therapy) as well as ≥ 1 high-risk feature including new ST changes, positive troponin levels, diabetes mellitus (DM), history of MI, peripheral artery disease, cerebrovascular disease, prior coronary artery bypass grafting (CABG) > 3 years ago, and multivessel coronary artery disease. Patients were excluded if they were undergoing CABG, their current statin therapy had potency > simvastatin 40 mg, their creatinine clearance was < 30 mL/min, or they had active liver disease.
The primary end point of the IMPROVE-IT trial was a composite score of CV death, MI, hospital admission for UA, coronary revascularization, or stroke [Blazing MA et al. Am Heart J. 2014]. Secondary end points included individual CV end points, as well as various composite scores. At baseline, the mean age was 64 years, 24.5% of patients were female, 27% had DM and 35.5% were on prior lipid-lowering therapy. In addition, the mean LDL-C level at the time of the ACS event was 95 mg/dL.
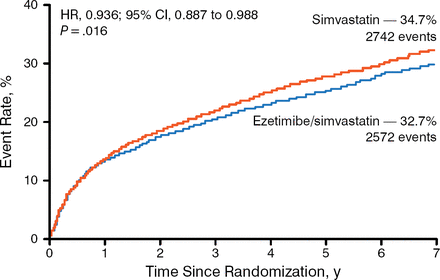
Treatment with ezetimibe plus simvastatin resulted in a greater decrease in mean LDL-C levels beginning at week 1 after randomization and remained steady up to 96 months. A significantly higher number of patients in the simvastatin monotherapy arm experienced the primary end point (34.7%) compared with patients in the ezetimibe plus simvastatin arm (34.7% vs 32.7%; HR, 0.936; 95% CI, 0.887 to 0.988; P = .016) with a number needed to treat (NNT) of 50 (Figure 1). Similarly, significantly fewer patients reached the composite of CV death, nonfatal MI, or nonfatal stroke in the ezetimibe plus simvastatin arm (20.4%) compared with the simvastatin arm (20.4% vs 22.2%; HR, 0.90; 95% CI, 0.84 to 0.97; P = .003) with an NNT of 56. In addition, fewer patients experienced the individual end points of MI and ischemic stroke in the combination therapy arm compared with simvastatin monotherapy.
Primary End Point of the IMPROVE-IT Trial
IMPROVE-IT, Improved Reduction of Outcomes: Vytorin (Ezetimibe/Simvastatin) Efficacy International Trial.
Reproduced with permission from CP Cannon, MD.
Similar rates of adverse events occurred among both arms, such as elevated liver enzymes, cholecystectomy, gallbladder-related events, rhabdomyolysis, myopathy, and cancer.
In conclusion, Dr Cannon stated that data from the IMPROVE-IT trial suggest that the addition of a nonstatin, LDL-C-lowering agent provides an additional clinical benefit beyond statin monotherapy. In addition, he commented that the results support the LDL hypothesis that lowering LDL-C can reduce the risk of CV events.
- © 2014 MD Conference Express®