Summary
The Dual Antiplatelet Therapy [DAPT] trial recruited patients from 5 studies conducted at 452 sites in 11 countries. The objective was to assess the efficacy and safety of dual antiplatelet therapy (DAPT) between 12 and 30 months in patients treated with coronary stents. This article discusses the primary results of the DAPT trial in the cohort treated with drug-eluting stents.
- interventional techniques & devices
- coronary artery disease
- cardiology clinical trials
The Dual Antiplatelet Therapy [DAPT; Mauri L et al. N Engl J Med. 2014] trial recruited patients from 5 studies conducted at 452 sites in 11 countries. Research institutions and stent manufacturers collaborated to conduct this trial at the request of the US Food and Drug Administration (FDA). The objective was to assess the efficacy and safety of dual antiplatelet therapy (DAPT) between 12 and 30 months in patients treated with coronary stents. Laura Mauri, MD, MSc, Brigham and Women's Hospital, Boston, Massachusetts, USA, presented the primary results of the DAPT trial in the cohort treated with drug-eluting stents (DESs).
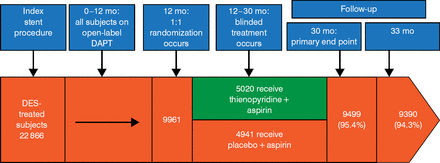
To study a broad patient population, there were few exclusion criteria. Patients treated with any FDA-approved DES or bare metal stent could enroll provided they were not taking oral anticoagulant therapy and had a life expectancy >3 years. After 12 months of open-label DAPT (aspirin plus thienopyridine), patients who had no myocardial infarction (MI), stroke, repeat revascularization, or moderate or severe bleeding and who were considered compliant with thienopyridine therapy were randomized to blinded treatment (aspirin plus thienopyridine or aspirin plus placebo). Randomization was stratified by site, DES versus bare metal stent, clopidogrel vs prasugrel, and presence of ≥ 1 stent thrombosis (ST) risk factors. The co-primary end points were definite or probable ST (Academic Research Consortium definition) and major adverse cardiac or cerebrovascular events (MACCEs; death, MI, or stroke). The primary safety end point was moderate or severe bleeding (GUSTO classification). Study design and patient disposition are presented in Figure 1.
Dual Antiplatelet Therapy Trial Design and Patient Disposition
DAPT, dual antiplatelet therapy; DES, drug-eluting stent.
Reproduced with permission from L Mauri, MD, MSc.
Of the 9961 patients randomized, 25% were women; the mean age was 61 years; 30% had diabetes mellitus; and 26% had evidence of MI at the index procedure. The stent lengths were relatively long (mean, 27 mm), and the left anterior descending artery was treated in 40% of patients. Half the patients had at least 1 ST risk factor—most commonly, presentation with MI (26%), lesion length ≥ 30 mm (10%), and bifurcation lesion (7%). Forty-seven percent of the patients were treated with everolimus-eluting stents, and 35% took prasugrel. The study results are presented in Table 1.
Dual Antiplatelet Therapy Study Results: Drug-Eluting Stent Patients 12 to 30 Months After Index Event
In the primary end point analysis, relative risk reductions of 71% for ST and 29% for MACCEs were seen in patients who continued thienopyridine treatment (P < .001). While all-cause mortality favored the placebo group, the number of cancer-related deaths in the continued therapy group (n = 31) was significantly higher than in the placebo group (n = 14; P = .02). When a sensitivity analysis was conducted after removal of the 9 patients whose deaths were related to cancers known to be present before enrollment in the study, all-cause mortality was no longer statistically significant (P = .14).
The benefit of prolonged thienopyridine therapy reduced both stent-related MI and MI occurring at other coronary locations. Subgroup analyses found that this treatment benefit was consistent across most subgroups. In addition, a small increase in total MI and ST was detected in the 30 days following discontinuation of thienopyridine therapy: both at 12 months and at 30 months. In her concluding remarks, Dr Mauri noted that whether the treatment benefits seen in the DAPT study will be generalizable to other types of stents or nonthienopyridine P2Y12 inhibitors has not been established.
- © 2014 MD Conference Express®