Summary
The Is There a Life for Drug Eluting Stent (DES) After Discontinuation of Clopidogrel trial [ITALIC/ITALICplus] was a large, prospective, open-label randomized trial conducted at 55 sites in Europe and the Middle East, and is discussed in this article. The objective of the trial was to determine if 6 months of dual antiplatelet therapy (DAPT) was noninferior to 24 months of DAPT following DES placement.
- interventional techniques & devices
The Is There a Life for Drug Eluting Stent (DES) After Discontinuation of Clopidogrel trial [ITALIC/ITALICplus; Gilard M et al. J Am Coll Cardiol. 2014] was a large, prospective, open-label randomized trial conducted at 55 sites in Europe and the Middle East. The objective of the trial was to determine if 6 months of dual antiplatelet therapy (DAPT) was noninferior to 24 months of DAPT following drug-eluting stent (DES) placement. Martine Gilard, MD, PhD, Brest University, Brest, France, presented the 12-month results of the ITALIC/ITALICplus trial.
Eligible patients had at least one Xience V DES placed and were pretreated with aspirin plus clopidogrel, prasugrel, or ticagrelor. Patients were not pretreated with abciximab during their hospital stay.
Patients were excluded if they had platelets < 100 000/μl, known hemorrhagic diathesis, major surgery during the previous 6 weeks or any scheduled during the year after enrollment, evidence of active gastrointestinal or urogenital bleeding, severe liver failure, a severe medical condition with a life expectancy < 2 years, or DES placement within the previous year, or if they underwent primary percutaneous coronary intervention (PCI) for acute ST elevation myocardial infarction (MI) or had treatment of the left main artery. Patients taking oral anticoagulation therapy and those who had contraindications to study drugs were also excluded.
The primary end point was a composite of death, MI, emergency target vessel revascularization, stroke, or major bleeding (TIMI criteria) within 12 months. Secondary end points included the same composite at 24 and 36 months, the individual components, and the incidence of TIMI minor and minimal bleeding. The study was stopped prematurely due to slow recruitment.
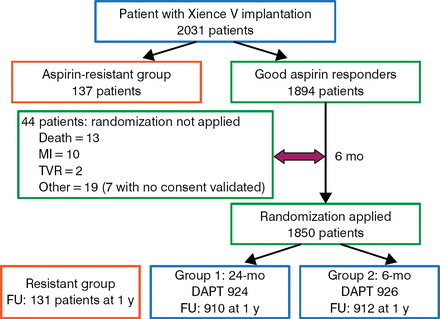
All patients were tested for aspirin resistance; 137 patients determined to be aspirin-resistant were not randomized. After 6 months of DAPT, patients who had no outcome-related events were randomized. A total of 1850 patients were randomized (n = 926 to 6 months of DAPT and n = 924 to 24 months of DAPT). Patient and procedural characteristics at baseline were similar between the 2 treatment arms. The study schematic and patient flow are illustrated in Figure 1.
Study Design and Patient Flow
DAPT, dual antiplatelet therapy; FU, follow-up; MI, myocardial infarction; pts, patients; TVR, target vessel revascularization.
Reproduced with permission from M Gilard, MD, PhD.
The mean age of the population was 62 years, 80% were men, and the mean body mass index was 27 kg/m2. Approximately 37% had type 2 diabetes, 15% had a previous MI, 23% had a previous PCI, and 5.8% had a previous coronary artery bypass graft. The PCI was considered a procedural success in > 98% of the patients randomized. Approximately 30% of patients had 2 lesions treated, and approximately 19% had ≥ 3 lesions treated. The majority of patients (> 98%) took clopidogrel.
There were no significant differences between the 2 arms at 12 months (Table 1). The noninferiority criteria were met between the 6-month and the 24-month DAPT groups (absolute risk difference, 0.11%; 95% CI, −1.04 to 1.26; P Noninferiority = 0002).
ITALIC/ITALICplus Study Results at 12 Months (Intent-to-Treat Population)
To conclude, Prof Gilard noted several limitations of this trial. The study had a small sample size due to its premature discontinuation; however, because we finally had a rate of events of 1.5% (compared to 3% expected), we might consider that the sample size would be enough to consider the conclusion as valid, also because we are far from the boundary.
- © 2014 MD Conference Express®