Summary
Lupus nephritis (LN) is a serious complication of systemic lupus erythematosus. This article discusses best practices in the diagnosis and management of patients with LN, as well as the use of oral steroids for the treatment of this disease.
- Lupus
- Rheumatology
- Lupus
Lupus nephritis (LN) is a serious complication of systemic lupus erythematosus (SLE). Michelle Petri, MD, MPH, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA, presented advice on how to diagnose and manage patients with LN. In Dr Petri's research program, LN is more common and more severe in men. To detect LN, it is often helpful to perform serological testing. Anti-C1q in combination with anti-double-stranded DNA antibodies and low complement levels has the strongest serological association with renal involvement [Orbai AM et al. Lupus. 2014].
However, the gold standard for measuring renal disease activity remains kidney biopsy (using the International Society of Nephrology [ISN] class I-VI, and the US National Institutes of Health [NIH] activity index and chronicity index). To assess renal activity clinically, the Systemic Lupus International Collaborating Clinics renal activity score is helpful. The score, derived from a regression analysis using the physician's rating of renal activity, is top-heavy on proteinuria data.
To detect LN, the gold standard is the 24-hour urine protein-to-creatinine ratio (UPCR) [Christopher-Stine L et al. J Rheumatol. 2004]. Spot UPCRs are acceptable in clinical practice.
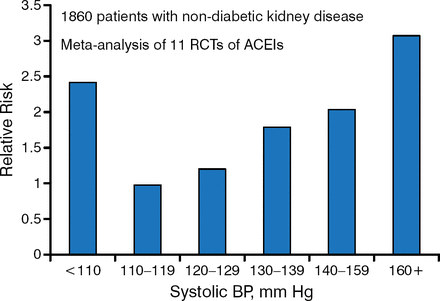
Once LN is detected, blood pressure (BP) control is key. A systolic BP goal between 110 and 129 mm Hg may be beneficial in patients with urine protein excretion > 1.0 g/d [Jafar JH et al. Ann Intern Med. 2003]. Systolic BP < 110 mm Hg may be associated with a higher risk for kidney disease progression (Figure 1).
Effect of Systolic Blood Pressure and Its Treatment in Chronic Kidney Disease
ACEI, angiotensin-converting enzyme inhibitor; BP, blood pressure; RCT, randomized clinical trial.
Source: Jafar JH et al. Ann Intern Med. 2003.
Reproduced with permission from Michelle Petri, MD, MPH.
BP lowering in patients with LN can be achieved effectively with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) [Pohl MA et al. J Am Soc Nephrol. 2005]. ACEIs and ARBs not only can be used to lower BP but also may function as renal-protective agents [Duràn-Barragàn S et al. Rheumatology (Oxford). 2008; Perna A et al. Kidney Int. 2000]. Patients with LN also should be treated with hydroxychloroquine (HCQ), Dr Petri said, because one study shows that it improves complete response rates with mycophenolate mofetil (MMF) [Kasitanon N et al. Lupus. 2006]. Although HCQ is frequently stopped in patients with LN, this study suggests it should be maintained.
Increasing 25-hydroxyvitamin D improves UPCR and the Physician's Global Assessment (PGA) score [Petri M et al. Arthritis Rheum. 2013]. Following vitamin D treatment, there was a 13% decrease in the odds of having a PGA score ≥ 1, a 21% decrease in the odds of having a Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score ≥ 5, and a 15% decrease in the odds of having a UPCR > 0.5.
SLEDAI and visual analog scale renal activity scores worsen during pregnancy. Mycophenolate, though, cannot be used in pregnancy because it causes fetal malformations. The recommendation is to continue prednisone and other treatments to management SLE flares during pregnancy but to never use MMF.
Ethnicity may affect MMF and intravenous (IV) cyclophosphamide treatment response. For example, black and Hispanic patients respond better to MMF than IV cyclophosphamide [Isenberg D et al. Rheumatology. 2010].
The ALMS maintenance trial [Dooley MA et al. N Engl J Med. 2011] showed MMF was significantly superior to azathioprine for maintaining a renal response and in preventing relapse in patients with LN who had a response to induction therapy (P = .003).
Dr Petri suggests that for the best response, the MMF dosing should be split, the dose should be adjusted for different ethnic groups, and the trough levels should be monitored. A recommended starting dose for MMF is 1000 mg BID. In the future, Dr Petri anticipates the use of multitarget therapies, adding biologics or a calcineurin inhibitor to MMF, tracking disease activity without renal biopsies, and new therapies to reduce renal fibrosis.
Liz Lightstone, MD, PhD, Imperial College London, London, United Kingdom, presented on her belief that oral steroid use could be replaced by rituximab for treating LN.
In the LUNAR trial [Rovin BH et al. Arthritis Rheum. 2012], rituximab plus MMF plus steroids was not superior to MMF plus steroids compared with the proportion of patients with LN who achieved a complete or partial response at week 52. A significant difference favoring rituximab was seen for some of the secondary end points such as a ≥ 50% reduction in proteinuria (P = .04) and complete-response or partial-response proteinuria (P = .04).
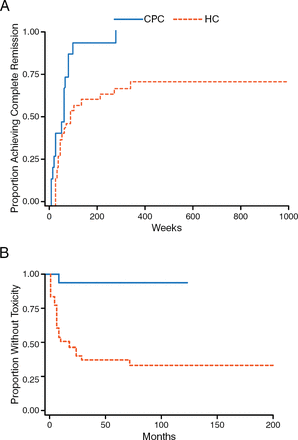
Long-term steroid use is problematic because it is associated with significant morbidity and premature mortality [Petri M. Lupus. 2000]. Recent studies have supported the use of lower doses of steroids being associated with improved or similar efficacy with fewer side effects. For instance, the Lupus group from Bilbao has shown that a combination of rapidly tapered oral prednisone, methylprednisolone (MP) pulses, and MMF or cyclophosphamide, always with HCQ, is more effective in achieving remission of LN than historical regimens containing high-dose prednisone (Figure 2) [Ruiz-Irastorza G et al. Autoimmun Rev. 2014]. There was less toxicity, better response, and fewer adverse events (AEs) with less steroid use.
Low-Dose Steroids Produce Better Response and Fewer Adverse Events
CPC, Cruces protocol cohort; HC, historic cohort.
Adapted from Ruiz-Irastorza G et al. Prednisone in lupus nephritis: How much is enough? Autoimmun Rev. 2014;13:206–214. Copyright (2014), with permission from Elsevier.
There is also evidence from renal transplant medicine that adding a biologic is safe, effective, and steroid sparing [Borrows R et al. Am J Transplant. 2004], although in transplantation the biologics have predominantly focused on anti-T cell effects. In LN, there are a lot of registry and case series data to support the use of rituximab in refractory LN. In one report, a complete or partial therapeutic response in predominantly refractory LN was achieved with rituximab in 67% of patients at 12 months [Díaz-Lagares C. Autoimmun Rev. 2012].
Dr Lightstone and her colleagues have gone a step further and developed the steroid-avoiding Rituxilup protocol. The initial results of a prospective cohort treated with rituximab, MP, and MMF but no oral steroids and followed for at least 1 year were published in 2013 [Condon MB et al. Ann Rheum Dis. 2013]. The regimen was used to treat biopsy-proven active ISN/Renal Pathology Society (RPS) class III, IV, or V LN, and it showed that oral steroids can be safely avoided when treating LN with Rituxilup. The Rituxilup regimen led to remission, preservation of renal function, and minimal oral steroid use in the majority of the 50 patients.
In the first 50 consecutive patients treated with 2 doses of rituximab (1 g) and MP (500 mg) with maintenance treatment with MMF alone, 90% of patients achieved complete biochemical remission (CR) or partial remission (PR) by a median time of 37 weeks (range, 4–200). By 52 weeks, CR and PR had been achieved in 52% (n = 26) and 34% (n = 17), respectively.
In a 5-year follow-up analysis from this study that included 42 patients, the majority (88%) achieved CR or PR. Median time to remission was 9 months with 77% never requiring oral steroids. AEs were similar to those in the initial study. The majority had preserved renal function.
To conclude, Dr Lightstone stated that predictors of poor outcomes in the Rituxilup cohort were baseline creatinine > 120 μmol/L and a < 50% reduction in proteinuria at 6 months. In addition, the minimal use of oral steroids in the majority would be expected to have long-term benefits in terms of cardiovascular disease risk and reduced side effects. The Rituxilup regimen will now be evaluated formally in a phase 3, open-label, multicenter, international, randomized controlled trial where the Rituxilup regimen will be compared with MMF and oral steroids [NCT01773616].
- © 2014 MD Conference Express®