Summary
In a session focusing on computer-assisted surgery, 6 presenters covered the new options for tools and techniques and the evidence for different approaches. Overall, these new tools and approaches can improve accuracy, reduce radiation exposure, potentially shorten operating times, and reduce costs.
- Orthopaedic Procedures Spine Conditions
- Orthopaedic Procedures
- Orthopaedics
- Spine Conditions
In a session focusing on computer-assisted surgery, 6 presenters covered the new options for tools and techniques and the evidence for different approaches. Overall, these new tools and approaches can improve accuracy, reduce radiation exposure, potentially shorten operating times, and reduce costs.
Russell H. Taylor, PhD, The Johns Hopkins University, Baltimore, Maryland, USA, discussed computer-assisted techniques, noting that while robotic tools have been used extensively in other surgical areas, their use in spine surgery is relatively new.
Dr Taylor emphasized that three components—humans, technology, and information—must interact smoothly for these new approaches to be successful. He first described the ROBODOC robot, developed > 20 years ago, which was used for joint replacement surgery based on computed tomography (CT) images [Taylor et al. IEEE Trans Rob Aut. 1994]. To illustrate emerging systems, he described experimental work combining cone-beam CT images with the da Vinci Surgical System and augmented reality displays to provide more information during transoral robotic surgery [Liu et al. J Robot Surg. 2013].
According to Dr Taylor, emerging computer-integrated surgery systems combine available information about a specific patient (eg, imaging) and general information based on statistics to develop a plan for the operating room. In the operating room, all of this information is “registered” to the physical patient. Once this is done, then the system can use appropriate technology to assist the physician to carry out the surgical plan and to perform postoperative assessments. The information generated in this process can be analyzed statistically to determine what is effective and appropriate, allowing improvements in treatment.
When using robots, it is possible to analyze and interpret movements of the machine. This can help improve treatment, and develop skills and training (eg, as a way to objectively assess the progress of residents and fellows).
According to Dr Taylor, while robots are not always necessary, their use in many cases can make surgery less invasive, safer, more consistent, more precise, and more cost-effective. In the future, all of the data available from robots and computer systems may be integrated with the hospital database to provide better outcomes and quality for improved patient care.
Michael MacMillan, MD, Southeastern Integrated Medical, Gainesville, Florida, USA, addressed current options available for computer-guided surgery. He first emphasized the importance of ensuring that the image of the spine and images of tools are working together accurately. A preoperative CT scan (ie, digital image of the spine) needs to be superimposed by physically touching the spine to see if there is a match with the image. This approach has been replaced by the use of fluoroscopic registration, which provides a high level of accuracy. A fluoroscopic image is created using the preoperative image and compared with a fluoroscopic image of the actual spine.
A synthetic fluoroscopic image—an image created from a preoperative digital image—has the advantage of faster registration and does not require landmarks to be exposed, making it suitable for minimally invasive surgery. However, it can sometimes be inaccurate and does not allow landmarks to be verified in real time (eg, if structures move during surgery or were positioned differently during the CT scan).
Intraoperative CT (in which the CT scan is taken in the operating room) has the advantage of allowing images to be taken while the patient is in position for surgery. However, sterility is a concern and this process disrupts the normal operative flow.
The new Mazor Robotics Renaissance Guidance System has a more nuanced approach because individual segments are examined, meaning that each vertebra has its own registration and preoperative plan. It improves accuracy, lowers costs, and does not require a camera that can obstruct visibility. This system does require a frame attached along the length of the spine, preoperative planning, and a drill guide on the attached frame. Additionally, it can only be used for pedicle screws at this time.
Srinivas K. Prasad, MD, Thomas Jefferson University, Philadelphia, Pennsylvania, USA, addressed the outcomes of current approaches in image-guided surgery. He began by emphasizing the importance of clarity when defining accuracy, as it can be used in different ways and can lead to a false sense of security. Additionally, even though many meta-analyses have been conducted, these have limitations such as variable accuracy assessment methods, heterogeneous patient populations and conditions, and disparate inclusion criteria for navigation. There also have been few prospective studies. Accuracy numbers may include a variety of patients and the direction of the breach often is not considered.
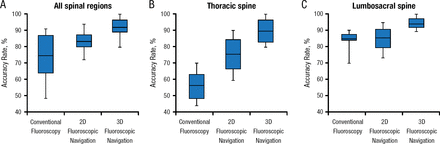
As new technologies have been developed, the level of accuracy has increased and tightened. Some studies suggest that certain regions and anatomical areas may be most sensitive to these new technologies. For example, one study indicated that accuracy rates improved the most with fluoroscopic vs conventional navigation in the thoracic region (Figure 1) [Mason et al. J Neurosurg Spine. 2014].
Accuracy Rate by Anatomical Region
2D, 2-dimensional; 3D, 3-dimensional.
Adapted from Mason A et al. The accuracy of pedicle screw placement using intraoperative image guidance systems. J Neurosurg Spine. 2014;20:196–203. With permission from American Association of Neurological Surgeons.
Accuracy is influenced by clinician experience. In one study, over time, computer-assisted navigation decreased the perforation rate and operative time [Bai et al. Chin Med J (Engl). 2010]. Despite this information on accuracy, there is insufficient information about how anatomy accuracy relates to patient benefits and outcomes.
Eric W. Nottmeier, MD, St. Vincent's Spine and Brain Institute, Jacksonville, Florida, USA, related data on the use of computer-assisted systems in spine surgery to cost-effectiveness. For health insurance company and hospital staff to support the use of these technologies, they must believe that the approaches are cost-effective. Marketing (as has been done for the da Vinci Surgical System) could increase patient demand. Additionally, these approaches may reduce operating room time, reduce mistakes and associated costs, and allow the use of standard (rather than cannulated) screws. Dr Nottmeier presented a theoretical model showing potential savings in the operating room and instrumentation savings, suggesting a potential annual savings of ≥ $500 000.
Bawarjan Schatlo, MD, University of Göttingen, Göttingen, Germany, presented details of the robotic systems currently available, including the ROSA Spine System and Mazor SpineAssist.
Dr Schatlo thoroughly discussed the Mazor Renaissance and SpineAssist robot family, which is mainly used to assist surgeons in placing pedicle screws. The former generation of this robot (SpineAssist) has now been in use for half a decade and therefore has been the focus of most of the presently available publications on robotic spine surgery. The reported accuracy rates of this system in clinical studies vary between 85% and 100%, which represents the proportion of screws with < 2-mm deviation from a perfect trajectory. Interestingly, most screw deviations observed in robot cases were lateral inaccuracies (70%) and as such were not deleterious to neural structures while lateral and medial misplacement in the freehand group were roughly equal [Ringel et al. Spine. 2012]. A preliminary but prospective study with a solid randomized design demonstrated an accuracy rate of approximately 97% with no cases of neurological injury and a decrease in radiation exposure [Roser et al. Neurosurgery. 2013]. One study suggested that physicians could expect to acquire proficiency in the use of the robotic guidance system after about 30 surgeries [Hu X, Lieberman IH. Clin Orthop Relat Res. 2014]. Dr Schatlo also noted that physicians who work with these systems should always remain prepared to switch to conventional techniques of pedicle screw insertion [Schatlo et al. J Neurosurg Spine. 2014].
He also briefly described the ROSA Spine System and the da Vinci Surgical System. The ROSA Spine System is the successor of the Neuromate and has a different concept from the Mazor SpineAssist. After intraoperative imaging and planning, the robot guides the surgeon in placing pedicle screws. Preliminary studies in Europe have shown promising results, but the system has not yet been approved by the US Food and Drug Administration. Dr Schatlo concluded by mentioning the da Vinci system (Intuitive Surgical), which has been used for anterior access to the lumbar spine [Beutler et al. Spine (Phila Pa 1976). 2013]. However, its utility for spinal applications has not yet been demonstrated in a prospective study.
Eric A. Potts, MD, Indiana University, Indianapolis, Indiana, USA, concluded the session by addressing the use of powered and automated instruments for spine surgery. Spine surgeons can develop injuries associated with repetitive movements [Auerbach JD et al. Spine (Phila Pa 1976). 2011] that can lead to missed work or early retirement.
Powered and automated instruments can result in improved accuracy, shorter operative times, and reduced radiation exposure. Dr Potts noted that it is relatively easy to translate skills into working with power equipment and that anecdotal data are encouraging. In the longer term, it may be possible to develop replacements for tactile feel.
- © 2015 MD Conference Express®