Summary
Hepatitis C virus genotype (GT)-3 is common worldwide and is accompanied by a significant disease burden. Among patients with cirrhosis, it is associated with increased risk of fibrosis progression, steatosis, and hepatocellular carcinoma. The Phase III Daclatasvir and Sofosbuvir for Genotype 3 Chronic HCV [ALLY-3; NCT02032901] trial evaluated the efficacy and safety of daclatasvir plus sofosbuvir in patients chronically infected with GT-3.
- Liver Conditions
- Viral Infections Hepatology Clinical Trials
- Liver Conditions
- Viral Infections
- Hepatology
- Hepatology Clinical Trials
Hepatitis C virus (HCV) genotype (GT)-3 is common worldwide and is accompanied by a significant disease burden [Pol S et al. Liver Int. 2014]. Among patients with cirrhosis, it is associated with increased risk of fibrosis progression, steatosis, and hepatocellular carcinoma [Nkontchou G et al. J Viral Hepat. 2011; Larsen C et al. J Med Virol. 2010; Bochud PY et al. J Hepatol. 2009]. Current treatment options are limited and require 24-week treatment that includes ribavirin. Results of the Phase III Daclatasvir and Sofosbuvir for Genotype 3 Chronic HCV [ALLY-3; NCT02032901] trial presented by David R. Nelson, MD, University of Florida, Gainesville, Florida, USA, show that oral therapy with the combination of daclatasvir (DCV) and sofosbuvir (SOF) achieves high rates of sustained viral response up to 12 weeks after therapy (SVR12).
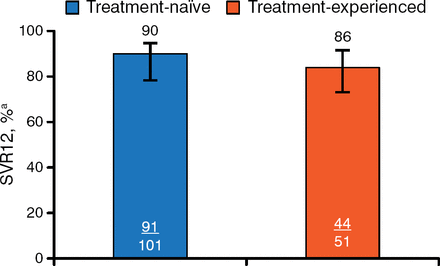
ALLY-3 was a phase 3 trial that evaluated the efficacy and safety of DCV plus SOF in patients chronically infected with GT-3. In this open-label trial, patients received oral DCV 60 mg plus oral SOF 400 mg twice daily for 12 weeks. The primary end point was SVR12 (HCV RNA < lower limit of assay quantification [LLOQ]). The study included treatment-naïve (n = 101) and treatment-experienced (n = 51) adult patients with chronic GT-3 infection and HCV RNA ≥ 10 000 IU/mL. Patients previously treated with NS5A inhibitors were excluded.
Overall, patients had a mean age of approximately 55 years. About two-thirds were men and the majority were white. Between 69% and 75% of the participants had a high viral load (> 800 000 IU/mL) and approximately 21% were cirrhotic as defined by the protocol. About 60% of the participants were non-CC IL28B GT. In the experienced group, about 61% of participants had relapsed, 14% had a null response, and 4% had a partial response. The cause of treatment failure in the remaining 22% was “other.” Seven patients in the experienced group had received prior treatment with SOF; 2 were treated with alisporivir.
Treatment with DCV plus SOF was associated with a high rate of SVR12 in both groups (Figure 1).
Primary End Point: Sustained Viral Response to Treatment 12 Weeks After Therapy
Error bars indicate 95% confidence intervals. SVR12, sustained viral response to treatment 12 weeks after therapy.
aHepatitis C virus RNA < lower limit of quantification (25 IU/mL).
Reproduced with permission from DR Nelson, MD.
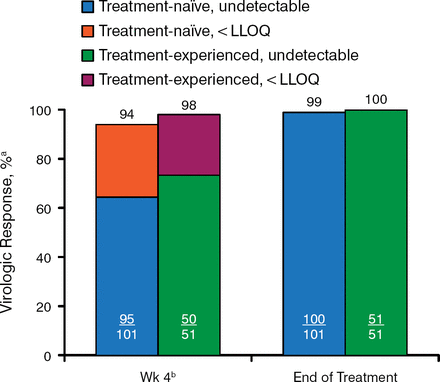
Figure 2 shows the on-treatment virologic response at week 4 and at the end of treatment for target not detected or target below the level of quantification. By the end of treatment, all but 1 patient were virus negative. Week-4 viral levels did not predict response vs nonresponse.
On-Treatment Virologic Response
HCV, hepatitis C virus; LLOQ, lower limit of quantification; SVR12, sustained viral response to treatment 12 weeks after therapy.
aUndetectable HCV RNA or HCV RNA < LLOQ (25 IU/mL).
bSVR12 rates based on week 4 HCV RNA levels: < LLOQ, target detected, 86%; < LLOQ, target not detected, 91%.
Reproduced with permission from DR Nelson, MD.
SVR12 rates were not influenced by sex, age < vs ≥ 65 years, HCV RNA levels, or IL28B polymorphism (CC vs non-CC). Cirrhosis did have a significant influence, regardless of prior treatment status. Overall, only 63% of patients with cirrhosis achieved SVR12 compared with 96% of patients without cirrhosis; rates were similar for treatment-naïve and experienced patients. Among patients with cirrhosis, 34% had portal hypertension (as assessed by platelet counts < 100 000/mm2). Sixteen patients relapsed; 11 of those had cirrhosis. The most common adverse events (> 10% of patients) were headache, fatigue, and nausea; none led to discontinuation.
The combination of DCV plus SOF was safe, well tolerated, and associated with SVR12 rates as high as 96% in patients without cirrhosis. There were no virologic breakthroughs. The resistance analysis for this study is ongoing.
- © 2014 MD Conference Express®