Summary
Intraocular pressure (IOP) is part of the pathogenesis of glaucoma. This article discusses treatment and management options for patients who are progressing at low levels of IOP, glaucoma medication, neuroprotective agents to preserve retinal ganglion cells, argon laser trabeculoplasty, prophylactic laser peripheral iridotomy, incisional glaucoma surgery, as well as minimally invasive glaucoma surgery.
- Ophthalmic Surgery
- Glaucoma
- Ophthalmology
- Ophthalmic Surgery
- Glaucoma
Intraocular pressure (IOP) is part of the pathogenesis of glaucoma. Target IOP should be part of the equation in the treatment of glaucoma, but it is not the whole story, according to Philip P. Chen, MD, University of Washington, Seattle, Washington, USA.
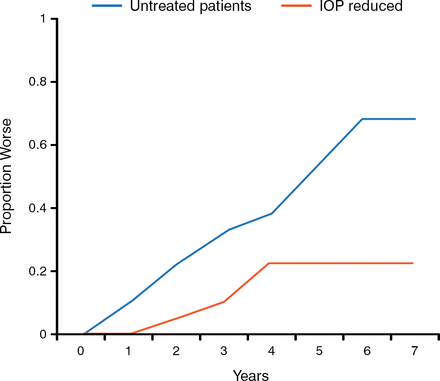
Target IOP is defined as the upper end of the IOP range at which glaucoma will be stable. Dr Chen concluded that reduced IOP can reduce the risk of visual field (VF) deterioration, citing several studies in which lower IOP is associated with reduced progression of VF defects (Figure 1) and better outcomes. He recommends starting with a 20% to 25% reduction in IOP, taking into consideration the patient's age and the severity and type of glaucoma. Target IOP should be viewed as a range and not a single value. The risk and burden should be weighed against the benefit; in some cases, it is reasonable to consider a less aggressive target, but in such a scenario the patient should be followed closely.
Reducing IOP Lowers the Risk of VF Worsening
IOP, intraocular pressure; VF, visual field.
Target 30% reduction in IOP in patients with normal tension glaucoma (actual 37%).
Data source: Collaborative Normal-Tension Glaucoma Study Group. Am J Opthalmol. 1998. Reproduced with permission from PP Chen, MD.
Ta Chen Chang, MD, Bascom Palmer Eye Institute, Miami, Florida, USA, discussed treatment and management options for patients who are progressing at low levels of IOP. Optimal management can be achieved via a checklist.
First, is the progression real? According to the Ocular Hypertension Treatment Study, 86% of VF abnormalities are not verified on retest. Ensure quality of retinal nerve fiber layer scan. Repeat the tests and confirm the progression. Is the apparent progression related to glaucoma? Perform neurologic and dilated fundus exams. Is the pressure really low? Compliance and lifestyle issues can affect pressure readings. Consolidate medications, reeducate the patient, and review lifestyle issues to determine whether laser or surgery is the best option. Does the rate of progression warrant the risk of intervention in lieu of disease severity, rate of progression, and life expectancy? The next issue is determining the risks and benefits of augmenting therapy. Medications and laser offer relatively low risk, while incisional surgery has relatively high risk. Lowering preoperative IOP has its own risk and benefit. Last, actively involve the patient in the treatment decision.
Peter A. Netland, MD, PhD, University of Virginia, Charlottesville, Virginia, USA, addressed the question of when and how the clinician should add or switch a glaucoma medication. Consider switching if monotherapy does not meet expectations with respect to efficacy or side effect profile. Alternatively, if monotherapy is working and patient is not at target IOP, consider adding a medication.
Patients may require improved IOP control or may develop side effects with current treatment. With a medical (vs surgical) approach to treatment, it is usually necessary to switch medications as opposed to adding a new one. Dr Netland recommends switching between or within drug class. Reasons to switch include achieving greater efficacy, avoiding adverse effects, or improving compliance. There is no universal regimen for switching vs adding a new medication. When adding therapy, many clinicians choose β-blockers, α2 agonists, and carbonic anhydrase inhibitors—and, although not recommended, many add fixed combination therapy. Decisions to switch or add medications should be evidence based and individualized for the patient.
Helen L. Kornmann, MD, PhD, Bascom Palmer Eye Institute, University of Miami, Miami, Florida, USA, explained which glaucoma medications can be safely used during pregnancy and in children.
In general, plan before pregnancy, or consider laser or incisional surgery. There is a paucity of safety data and a lack of information about relative risks. However, there are new rules to improve risk-benefit assessment for pregnancy and lactation labeling. These include a risk summary, clinical considerations, and data about pregnancy and lactation.
β-Blockers (US Food and Drug Administration class C) can cross the placenta to create fetal bradycardia and arrhythmia and can be secreted into breast milk, with some reports of apnea in neonates. β-Blockers can also be used until shortly before delivery, but topical gels should be considered a safer alternative. Carbonic anhydrase inhibitors (class C) are well tolerated during pregnancy and lactation with little evidence of adverse effects. Prostaglandin analogs (class C) increase uterine contractility and decrease blood flow in the fetus and thus are not recommended as a first-line treatment. α2 Adrenergic agonists (class B) are well tolerated during pregnancy but should be discontinued before delivery and avoided during lactation. There have been some reports of hyperthermia, seizures, or restlessness in neonates with miotic (class C) use. Although used safely during pregnancy for generations, miotic agents should not be used during lactation.
As for children, the US Food and Drug Administration has established the safety of β-blockers in pediatric patients < 6 years of age. Oral carbonic anhydrase inhibitors are well tolerated and effective at doses between 8 and 30 mg/kg/d in infants. Latanoprost is the only prostaglandin analogue with data reported in the pediatric population. It is safe and efficacious in older pediatric patients. α2 Agonists are highly lipophilic and easily pass through the blood-brain barrier. Side effects are more frequent than with other medications. They are contraindicated in children < 2 years and not recommended in children < 20 kg or < 6 years.
Louis R. Pasquale, MD, Harvard Medical School, Boston, Massachusetts, USA, reviewed the use of neuroprotective agents to preserve retinal ganglion cells in patients with preexisting glaucomatous optic neuropathy.
Neuroprotection is defined as averting progressive neuronal loss in patients with preexisting glaucomatous optic neuropathy. As opposed to neuroprevention, which takes years to decades to accomplish, or neurorejuvenation, which can be done in minutes, neuroprotection takes months to years. Citing the Early Manifest Glaucoma Trial, Dr Pasquale noted that topical β-blocker and laser trabeculoplasty prevented disease progression (reduced IOP) in patients with open-angle glaucoma [Heijl A et al. Arch Ophthalmol. 2002]. In another neuroprotection trial, the Low-Pressure Glaucoma Treatment Study, patients with low-pressure glaucoma (IOP < 22 mm Hg) who were treated with topical brimonidine (an α2 adrenergic agonist) were less likely to experience VF progression as compared to patients treated with timolol maleate (β adrenergic antagonist) [Krupin T et al. Am J Ophthalmol. 2011]. Several reasons were given for the effectiveness of brimonidine in this study. The brimonidine likely reached the optic nerve at levels needed to exert a neuroprotective effect. However, the study may have been overpopulated by patients with IOP-independent mechanisms of glaucomatous optic neuropathy (eg, vascular dysregulation). Brimonidine also corrects retinal vascular dysregulation and reduces disc hemorrhages, a biomarker for VF progression. Suggesting that we perhaps do not lower IOP enough, Dr Pasquale noted that lower mean IOP during treatment, a lower minimum IOP, and lower sustained levels of IOP during follow-up are associated with VF improvement [Musch DC et al. Am J Ophthalmol. 2014].
Kuldev Singh, MD, MPH, Stanford University School of Medicine, Stanford, California, USA, made a case for an association between inadequate disease surveillance and glaucoma progression. Some of the key issues faced by clinicians and patients include low awareness of disease presence, no treatment or undertreatment, poor compliance with treatment, and variability in the glaucoma natural history. Lowering IOP does not always eliminate risk, and a small percentage of patients actually go blind, posing additional challenges in disease surveillance and management. In addition, dogma sometimes gets in the way of facts. Disease progression is not always predictable, and compliance or noncompliance with medication use is not always related to outcome.
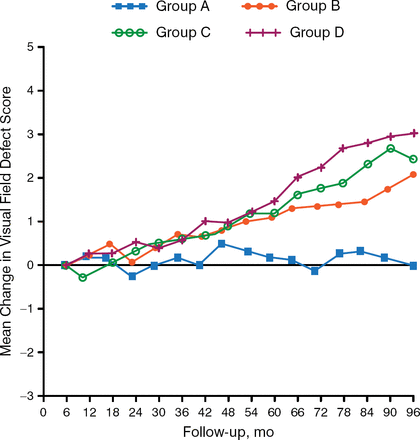
The Advanced Glaucoma Intervention Study noted an association between low IOP and reduced progression of VF defect. In addition, patients with IOP < 18 mm Hg who were 100% compliant with follow-up had a 0% mean change from baseline in VF defect score; patients with a 50% follow-up compliance rate had a VF defect score that worsened by 0.63 units [AGIS Investigators. Am J Ophthalmol. 2000; Figure 2].
Effect of Follow-up Compliance on Visual Field Worsening
Adapted from American Journal of Ophthalmology, 130, AGIS Investigators, The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration, 429–440, Copyright 2000, with permission from Elsevier.
In another study [Ung C et al. Ophthalmology. 2013], severity of illness was a driver of compliance—patients with severe glaucoma were more likely to adhere to their topical IOP-lowering medication regimen versus those with milder glaucoma. However, those with severe glaucoma were less likely to attend follow-up visits, suggesting that poor follow-up compliance leads to worsening of glaucoma [Ung C et al. Am J Ophthalmol. 2013]. Dr Singh concluded that compliance with therapy is affected by disease severity and that inadequate disease surveillance might be a risk factor for poor outcomes.
The Glaucoma Laser Trial suggested that primary argon laser trabeculoplasty (ALT) was a reasonable first-line treatment. Don Budenz, MD, MPH, UNC School of Medicine, Chapel Hill, North Carolina, USA, said that selective laser trabeculoplasty (SLT) may be as effective as an additional medication in lowering IOP and is comparable to ALT. SLT is often used as initial or adjunctive therapy for open-angle glaucoma in patients with poor compliance issues and in patients with fluctuating IOP. As a primary therapy, SLT provided similar IOP reductions (∼ 30%) when compared with medication (latanoprost) for patients with open-angle glaucoma and ocular hypertension (OH) over 12 months [McLiraith I et al. J Glaucoma. 2006]. SLT also had similar efficacy in other types of glaucoma, and up to 5 years after surgery, it produced equivalent IOP reductions, with less concern about side effects and patient compliance.
Many clinicians incorporate SLT as add-on therapy when medications inadequately control IOP. In one study, 70% of patients treated with add-on SLT had an IOP reduction of ≥ 3 mm Hg after maximal medical therapy. A number of studies comparing ALT and SLT support the use of add-on SLT therapy, with overall success rates for IOP reduction over a 5-year period somewhat (not significant) better with SLT.
Indications for prophylactic laser peripheral iridotomy (LPI) were reviewed by Douglas J. Rhee, MD, University Hospitals Eye Institute, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA. Preventive laser iridotomy is effective for preventing acute angle-closure attack in the fellow eye; it is probably effective in preventing an attack in an eye with a potentially occludable angle and may also be effective in eyes with narrow angles. In primary angle-closure suspects, LPI can prevent progression to primary angle-closure glaucoma [Pandav SS et al. Can J Ophthalmol. 2007]. When plateau iris syndrome is present, argon LPI is highly effective in eliminating residual appositional closure after laser iridotomy [Ritch R et al. Ophthalmology. 2014]. Prophylactic treatment with LPI does not prevent exercise-induced or acutely affected IOP rise in patients with pigment dispersion syndrome (PDS), nor does it prevent progression from PDS to pigmentary glaucoma in patients with OH [Scott A et al. Ophthalmology. 2011].
Phacomorphic glaucoma is an angle-closure glaucoma that directly results from a mature or hypermature lens that has become intumescent. Medical management includes preoperative mannitol, lens removal, and possibly LPI before subsequent cataract extraction. Prognosis is often poor, with only 60% having better than 20/40 vision.
Joseph F. Panarelli, MD, School of Medicine at Mount Sinai, New York, New York, USA, discussed perioperative considerations for patients who are candidates for incisional glaucoma surgery. The future benefits of the surgery must be weighed against the risks, and patients must be individualized. Factors to consider include patients' progression or likely progression of glaucoma, its rate, their age, and the likelihood of vision loss or vision reduction.
Microinvasive glaucoma surgery is Dr Pannarelli's preferred approach, as it is an efficient procedure that results in less trauma and has a rapid recovery and good safety profile. Medical and surgical treatments have similar outcomes regarding loss of visual acuity, but some data suggest that operating earlier may be beneficial. The Collaborative Initial Glaucoma Treatment Study showed that patients with advanced VF deficit who received early surgery did better than those treated with medication. A number of studies have shown good surgical outcomes without serious complications.
Too often, however, patients do not understand or see the need for surgery. Before surgery is considered, the patient's age, ocular surgical history, and family history should be taken into account. Individual decisions should be based on the patient's IOP, VF defect, and optic nerve condition. The “go” decision is when the risk of continuing to observe outweighs the risk of surgery.
Malik Y. Kahook, MD, University of Colorado School of Medicine, Aurora, Colorado, USA, discussed the role of minimally invasive glaucoma surgery (MIGS), defined as any surgical manipulation or device implantation, combined with cataract surgery through a self-sealing incision, that results in sustained IOP decrease. Cataract surgery alone may not produce the achieved goals; thus, there is a need for a cost-efficient procedure that is as effective as trabeculectomy and easy to perform with fewer postoperative effects. The Glaukos iStent® was the first MIGS implant.
The iStent is inserted through a small temporal clear corneal incision and is placed in the Schlemm canal at the lower nasal quadrant. This allows aqueous humor to drain directly from the anterior chamber into the Schlemm canal, bypassing the obstructed trabecular meshwork. The US IDE trial with the iStent, in combination with cataract surgery, reported that 72% of iStent-treated eyes vs 50% of control eyes achieved significant (P < .001) IOP reduction (≤ 21 mm Hg) at 1 year [Samuelson TW et al. Ophthalmology. 2011]. In each case, various factors must be considered (IOP, cataracts, safety, risk factors, etc) to strike a balance between risk and IOP lowering. Dr Kahook recommends using MIGS with glaucoma surgery in patients with a target IOP in the midteens, when trabeculectomy or glaucoma drainage devices are contraindicated and if there are good social reasons (eg, patient's job).
“Which is better: tube or trabeculectomy?” According to Dale K. Heuer, MD, Eye Institute, Medical College of Wisconsin, Milwaukee, Wisconsin, USA, the answer depends on the outcome measured. For instance, after 5 years, IOP reduction and changes in visual function were similar in 2 studies comparing these procedures; however, the number of patients requiring reoperation for glaucoma was significantly greater in the trabeculectomy group, as was the failure to achieve target IOPs (P = .002) [Wilson MR et al. Am J Ophthalmol. 2003; Gedde SJ et al. Am J Ophthalmol. 2012]. Medication use for glaucoma was greater (P < .001) for shunt use at 1 year but similar at 3 and 5 years. Complication rates were similar for the 2 procedures in the Wilson study. In the Gedde study, early wound leak, late bleb leak, and dysesthesia were significantly greater (P < .02) in the trabeculectomy group. Overall costs for the tube procedure are slightly higher than those for trabeculectomy. Quality-of-life outcomes for the 2 procedures remain unanswered. However, since 1994 the number of shunt procedures has increased while trabeculectomy procedures have decreased.
- © 2014 MD Conference Express®