Summary
This article reviews the main data regarding ticagrelor, prasugrel, and clopidogrel therapy in patients with ST-segment elevation myocardial infarction (STEMI). Additional topics include new evidence regarding the timing of antiplatelet loading, managing antiplatelet therapy in patients undergoing CABG surgery, as well as new strategies for managing atrial fibrillation and concomitant coronary artery disease and acute coronary syndromes.
- Myocardial Infarction
- Coronary Artery Disease
- Interventional Techniques & Devices
- Arrhythmias
- Myocardial Infarction
- Coronary Artery Disease
- Interventional Techniques & Devices
- Cardiology
- Arrhythmias
Pierluigi Tricoci, MD, MHS, PhD, Duke University Medical Center, Durham, North Carolina, USA, reviewed the main data regarding ticagrelor, prasugrel, and clopidogrel therapy in patients with ST-segment elevation myocardial infarction (STEMI).
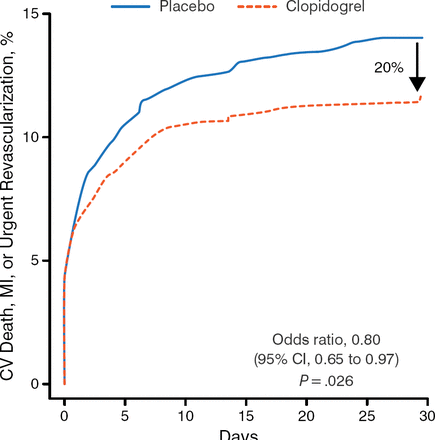
The CLARITY trial [Sabatine MS et al. N Engl J Med. 2005] established that the addition of clopidogrel to aspirin plus fibrinolytics improves the patency rate of the infarct-related artery and reduces ischemic complications (Figure 1). This study also reported benefit with clopidogrel pretreatment, despite its slower onset of action vs other antiplatelet therapies [Sabatine MS et al. JAMA. 2005].
Clopidogrel vs Placebo in Patients with STEMI
CV, cardiovascular; MI, myocardial infarction.
Adapted from New Engl J Med. Sabatine MS et al, Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation, 352, 1179–1189. Copyright © 2005. Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Shortcomings of clopidogrel include a delayed bio-availability in patients with STEMI [Heestermans AA et al. Thromb Res. 2008] and genetic variability that affects metabolism and platelet function [Holmes MV et al. JAMA. 2011]. Some evidence suggests that doubling the dose may help overcome these negative qualities [Mehta SR et al. N Engl J Med. 2010]. Advantages for prasugrel over clopidogrel include less susceptibility to genetic variation and drug-drug interactions, and more rapid, more consistent, and higher levels of platelet inhibition [Wiviott SD et al. Circulation. 2010]. The TRITONTIMI 38 trial [Wiviott SD et al. N Engl J Med. 2007] showed that prasugrel was more effective than clopidogrel in reducing cardiovascular (CV) death, MI, stroke, and stent thrombosis but at a cost of increased bleeding.
Ticagrelor can achieve a faster and longer antiplatelet effect compared with clopidogrel, and its maintenance dose sustains greater platelet inhibition throughout time [Storey RF. Eur Heart J Suppl. 2008]. Compared with clopidogrel, ticagrelor leads to greater reductions in mortality, MI, stroke, and stent thrombosis without increasing the total major bleeding risk [Wallentin L et al. N Engl J Med. 2009]. The platelet inhibition achieved with ticagrelor is similar to that of prasugrel [Parodi G et al. J Am Coll Cardiol. 2013].
Both ticagrelor and prasugrel have been shown to be superior to clopidogrel, and they should be the agents of choice in patients with STEMI who are treated with percutaneous coronary intervention (PCI). In the PLATO trial, ticagrelor also reduced CV death.
Steen Husted, MD, DSc, Medical Department, Hospital Unit West, Herning, Denmark, presented new evidence regarding the timing of antiplatelet loading in patients presenting with acute MI.
The 2014 European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines on myocardial revascularization [Windecker S et al. Eur Heart J. 2014] recommend that patients undergoing PCI receive a combination of aspirin plus a P2Y12 receptor blocker as early as possible before angiography and a parenteral anticoagulant to lower the risk of stent thrombosis.
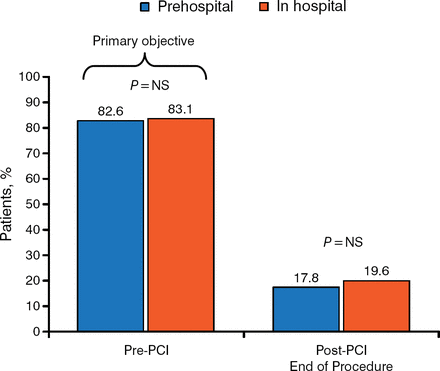
Pretreatment (prior to arrival at the hospital or upstream of coronary angiography) is appropriate for patients with STEMI because the diagnosis is often clear, the risk of urgent surgery is low, and oral antiplatelet therapy requires several hours to reach efficacy. The ATLANTIC study [Montalescot G et al. N Engl J Med. 2014] assessed outcomes following antiplatelet therapy administered in the ambulance compared with in-hospital administration (time difference of 31 minutes) in patients having STEMI with intended primary PCI. Coprimary end points were the percentage of patients reaching TIMI flow grade 3 in the infarct-related artery at initial angiography or achieving ≥ 70% STE resolution pre-PCI. There was no significant difference in the components of the primary end point (Figure 2), or in the occurrence of major adverse cardiac events (MACEs) or non-coronary artery bypass graft (CABG)-related bleeding events.
No TIMI Flow Grade 3 in Infarct-Related Artery
NS, nonsignificant; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Data source: Montalescot G et al. N Engl J Med. 2014. Reproduced with permission from S Husted, MD, DSc.
There was, however, a significant reduction (P = .0225) in definite stent thrombosis up to 30 days.
Administration of prehospital ticagrelor shortly before PCI in patients with ongoing STEMI is safe and reduces the risk of post-PCI stent thrombosis; it does not improve pre-PCI coronary reperfusion. In patients with non-STE acute coronary syndrome (NSTE-ACS), in which diagnostic uncertainty is frequent, Dr Husted recommends starting therapy with ticagrelor when diagnosis is confirmed.
Pretreatment with clopidogrel significantly (P < .001) reduces the absolute risk of MACEs but not death in patients undergoing PCI (STEMI and NSTE-ACS) [Bellemain-Appaix A. JAMA. 2012]. Clopidogrel may be useful in patients waiting for angiography. In the ACCOAST trial [Montalescot G et al. N Engl J Med. 2013], prasugrel at the time of diagnosis compared with after angiography (time difference, 4 hours) in patients with NSTE-ACS did not reduce any of the primary outcome factors, but it significantly (P = .006) increased the rate of TIMI major bleeding. As a result, prehospital treatment of patients with NSTE-ACS with prasugrel is not supported by the current data. In patients with NSTE-ACS, pretreatment before angiography may be beneficial if patients have to wait for the angiography or if the P2Y12 agent used has a slow onset of action.
Sven Wassmann MD, Department of Cardiology, Isar Heart Center, Munich, Germany, previewed new strategies for managing atrial fibrillation (AF) and concomitant coronary artery disease (CAD) and acute coronary syndromes (ACSs).
Patients with AF with CAD, stents, or ACS often require treatment with both antiplatelet and anticoagulant therapy to reduce CV events and the risk of stroke. This approach, however, significantly increases the incidence of major bleeding, with longer duration of therapy being associated with increased risk [Hansen ML et al. Arch Intern Med. 2010]. Thus, reducing the time of triple therapy is an important step. The 2012 focused update of the ESC guidelines for the management of AF [Camm AJ et al. Eur Heart J. 2012] recommends treating with triple therapy in the early phase followed by dual therapy in the later phase (after 6 months) in patients with low bleeding risk. After 12 months, only anticoagulation treatment should be used. In patients with high bleeding risk, the time of triple therapy can be reduced to 1 month.
The ISAR-TRIPLE trial [NCT00776633] compared triple therapy for 6 months versus 6 weeks and found no significant (P = .63) difference regarding the combined end point of death, MI, stent thrombosis, stroke, or major TIMI bleeding [Sarafoff N et al. TCT 2014].
Using dual therapy from the start is another approach. The WOEST study [Dewilde WJ et al. Lancet. 2013] reported that the use of a vitamin K antagonist (VKA) oral anticoagulant (OAC) plus clopidogrel (dual therapy) significantly (P < .0001) reduced bleeding compared with triple therapy (OAC plus clopidogrel plus aspirin) with no increase in the rate of thrombotic events in stented patients with AF.
Non-VKA OACs appear to be associated with a lower bleeding risk, particularly at low doses [Ruff CT et al. Lancet. 2014]. In a subset analysis of the RE-LY trial [Dans Al et al. Circulation. 2013], concomitant antiplatelet drugs appeared to increase the risk for major bleeding without affecting the advantages of dabigatran over warfarin. Dabigatran, however, appeared to have a lower absolute risk of bleeding with lower doses.
Current joint guidelines on the management of patients with AF and ACSs/stents [Lip GY et al. Eur Heart J. 2014] provide new guidance on treating these patients with OACs and antiplatelet therapy (Table 1). Definitive clinical studies to document these consensus statements are in process.
ESC/EHRA/EAPCI/ACCA/APHRS 2014 Consensus Document for Management of Antithrombotic Therapy in Patients With Atrial Fibrillation
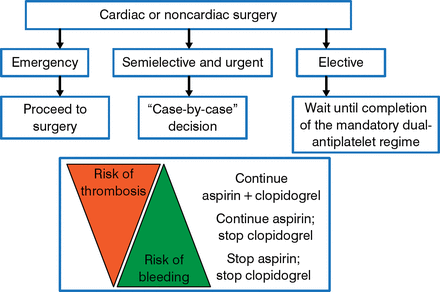
In patients with NSTE-ACS, the ESC/EACTS guidelines recommend a revascularization strategy based on the individual patient's clinical status and disease severity (Figure 3) [Windecker S et al. Eur Heart J. 2014].
Algorithm for Preoperative Management of Patients Under Dual Antiplatelet Therapy
Windecker S et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2014;35:2541–2619. With permission from European Society of Cardiology.
Piroze M. Davierwala, MD, Heart Center, University of Leipzig, Leipzig, Germany, discussed managing antiplatelet therapy in patients undergoing CABG surgery.
Treatment with single and dual antiplatelet therapy (DAPT) affects early and late outcomes, but despite multiple studies [Au AG et al. Am J Med. 2012; Smith PK et al. J Am Coll Cardiol. 2012; Held C et al. J Am Coll Cardiol. 2011; Nijjer SS et al. Eur Heart J. 2011; Bybee KA et al. Circulation. 2005], there is still no definitive answer concerning the safety of DAPT in the context of CABG. Nor is the optimal bleeding-thrombotic risk balance known. Ongoing trials may help to answer these questions.
Prevention of bleeding is important because bleeding and/or blood transfusion is independently associated with an increased risk of serious complications and death [Koch CG et al. Ann Thorac Surg. 2006; Rao SV et al. Am J Cardiol. 2005]. All patients should be assessed for bleeding risk, and preoperative interventions that may reduce the risk of bleeding should be taken. Proper management of antiplatelet therapy prior to surgery is critical (Figure 3) and should be a multidisciplinary effort [Sousa-Uva M et al. Eur Heart J. 2014]. Bridging strategies should be adopted for patients at high risk for thrombosis [Sousa-Uva M et al. Eur Heart J. 2014]. It is reasonable to use platelet function monitoring to determine the timing of surgery rather than an arbitrary period of delay, although this has not yet been tested prospectively [Janssen PW et al. Blood Rev. 2014].
In general, more attention should be directed to blood conservation and the judicious use of red blood cells. Off-pump CABG, small circuits, antifibrinolytic drugs, erythrocyte salvage devices (cell savers), and meticulous surgical hemostasis and technique can all contribute to reduce blood loss.
After CABG, all patients should receive aspirin, and patients with ACS should continue on DAPT (aspirin plus ticagrelor or clopidogrel) for 12 months.
- © 2014 MD Conference Express®