Summary
During this session, experts discussed various approaches to the management of new-onset atrial fibrillation (AF). Specific topics include AF in patients presenting with myocardial infarction, cardioversion therapy, anticoagulation therapy, as well as patients with minimal symptoms.
- Arrhythmias
- Cardiology
- Arrhythmias
During this session, experts discussed various approaches to the management of new-onset atrial fibrillation (AF). Antonio Raviele, MD, Alliance to Fight Atrial Fibrillation, Mestre, Venice, Italy, opened the session with a discussion of AF in patients presenting with myocardial infarction.
Despite the widespread use of reperfusion and pharmacologic therapies, AF remains a commonly encountered complication of acute myocardial infarction (AMI) that is associated with an excess risk of reinfarction, heart failure (HF), stroke, and mortality [Jabre P et al. Circulation. 2011]. Several possible mechanisms for, and predictors of, the development of AF in the presence of AMI have been identified (Tables 1 and 2). Once AF develops, there is usually a significant worsening of hemodynamics, owing to high ventricular rate, irregular ventricular filling, and loss of the atrial contribution to cardiac output.
Potential Mechanisms for AF in Patients With AMI
Predictors of AF in Patients With AMI
In many cases, the arrhythmia is well tolerated, and no additional treatment is required, as these patients commonly receive rate control and antithrombotic therapy for the index AMI. However, in some patients, the high ventricular rate associated with AF may contribute to hemodynamic impairment and the development of HF and thus require intervention with diuretics, additional rate or rhythm agents, and/or cardioversion.
According to the most recent position paper from the Joint European Heart Rhythm Association, Acute Cardiovascular Care Association, and European Association of Percutaneous Cardiovascular Interventions Task Force, adequate rate control represents the most important first therapeutic approach in patients with AMI with AF and rapid ventricular response [Gorenek B et al. Europace. 2014]. In stable patients, β-blockers or calcium channel blockers are recommended to reduce high ventricular rate. In patients with severe left ventricular dysfunction or HF, intravenous amiodarone and/or digitalis are recommended [Camm AJ et al. Eur Heart J. 2010]. Acute initiation of rate control therapy should be followed by a long-term strategy. Although few data are available, the consensus indicates that the optimal level of rate control in patients with AF and AMI should be 80 to 100 beats per minute (bpm). When adequate rate control cannot be achieved, urgent cardioversion is required, especially in patients with severe hemodynamic instability or intractable ischemia.
According to Michiel Rienstra, MD, PhD, University Medical Center, Groningen, The Netherlands, electrical cardioversion is more effective than pharmacologic cardioversion, and it is the treatment of choice in unstable patients. There are, however, advantages to a pharmacologic approach: It does not require sedation, and successful in-hospital treatment can provide guidance on the antiarrhythmic choice for ongoing management. The choice of antiarrhythmic agent should be based on the duration of the AF and the presence of structural heart disease.
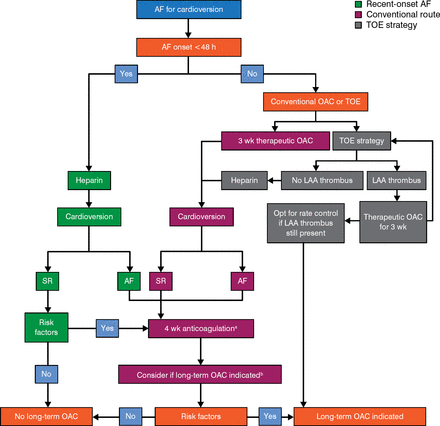
Over time, AF generally progresses to a permanent form. The management of new-onset AF begins with a stroke risk assessment, followed by an assessment of the need for oral anticoagulant therapy and rate control therapy. Elective electrical cardioversion may also be considered to prevent atrial remodeling [Camm AJ et al. Eur Heart J. 2010]. Silvia Zagnoni, MD, Ospedale Maggiore, Bologna, Italy, discussed strategies for anticoagulation therapy (Figure 1) in the setting of cardioversion.
Anticoagulation Protocol for Cardioversion
AF, atrial fibrillation; LAA, left atrial appendage; OAC, oral anticoagulants; SR, sinus rhythm; TOE, transesophageal echocardiogram.
aAnticoagulation should normally be continued for 4 weeks after a cardioversion attempt except when AF is recent onset and no risk factors are present.
bLong-term OAC if stroke risk factors and/or risk of AF recurrence/presence of thrombus.
Adapted from Camm AJ et al, Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC).
Eur Heart J. 2010;31:2369–2429. With permission from European Society of Cardiology.
For patients with AF of > 48-hour duration (or if the duration is unknown), current guidelines call for the use of either vitamin K antagonists (VKAs) with a target international normalized ratio of 2 to 3 or dabigatran for ≥ 3 weeks prior to and ≥ 4 weeks after cardioversion [Camm AJ et al. Eur Heart J. 2012]. In patients with risk factors for stroke or AF recurrence, oral anticoagulation therapy should be continued lifelong.
Although multiple randomized placebo-controlled trials have confirmed novel oral anticoagulants (NOACs) to be as effective as VKA, there is less evidence confirming their efficacy in the setting of cardioversion, derived mainly from post hoc analysis of performed cardioversions during the NOAC trials and observational small registries. Nowadays, the available data on the use of a NOAC in the setting of cardioversion after 3 weeks of anticoagulation derive from a single randomized trial: X-VeRT [Ezekowitz MD et al. Am Heart J. 2014]. Since compliance with NOAC use cannot be confirmed with a laboratory test, patients must be explicitly asked about their levels of compliance preceding a cardioversion; if there is any uncertainty concerning a patient's compliance, a transesophageal echocardiogram should be considered to rule out left atrial appendage thrombus.
Michael Glikson, MD, Davidai Arrhythmia Venter, Heart Institute, Sheba Medical Center, Tel Hashomer, Israel, discussed the management of new-onset AF in patients with minimal symptoms. Current European Society of Cardiology guidelines call for screening of AF in patients aged ≥ 65 years via pulse taking, followed by an electrocardiogram (class A; level of evidence B) [Camm AJ. Eur Heart J. 2012]. Subtle symptoms that may be indicative of HF should also be identified (eg, as evaluated through an European Heart Rhythm Association score). Consideration should be given to whether anticoagulation is recommended for thromboembolic risk reduction (ie, CHA2DS2-VASc score ≥ 1), bleeding risk considerations (HAS-BLED), and current level of rate control. Basic evaluation tools are show in Table 3.
Basic Tools for the Evaluation of Atrial Fibrillation
With certain exceptions (Table 4), rate control with anticoagulation is the preferred approach in most elderly patients with asymptomatic AF (class I; level of evidence A) [Camm AJ et al. Eur Heart J. 2010]. Based on data from the RACE II study—which found no difference in clinical outcomes [Van Gelder IC et al. N Engl J Med. 2010] or quality of life [Groenveld HF et al. J Am Coll Cardiol. 2011] between lenient rate control (resting rate < 110 bpm) and strict rate control (rest < 80 bpm; moderate exercise < 110 bpm)—Prof Glikson and the guidelines recommend lenient rate control for patients with asymptomatic AF.
Patients for Whom a Rhythm Control Strategy Is Preferred
About 20% to 30% of elderly patients with a cardiovascular implantable electronic device (CIED) and no prior diagnosis of AF have evidence of AF on device interrogation. Several studies have assessed the prognostic and therapeutic significance of this finding in an attempt to determine whether there is a critical duration or burden of AF that justifies anticoagulation, but the results have been inconsistent. Prof Glikson recommends that in patients with a CHADS score of 0, no treatment is needed (ie, no aspirin or antithrombotic); however, patients with a CHADS score of 1 or 2 should be treated if the AF duration is > 24 hours, while those with a CHADS score > 2 should be treated if the duration is > 5 or 6 minutes [De Cicco AE et al. Heart Rhythm. 2014].
Whether CIED AF alerts might be useful in improving outcomes and whether the above approach applies to patients with paroxysmal AF detected without a CIED (ie, with routine rhythm monitoring) has yet to be determined.
- © 2014 MD Conference Express®