Summary
Patients with non-ST-elevation (NSTE) acute coronary syndrome (ACS) are treated with antiplatelet and anticoagulation to reduce ischemic complications [Hamm CW et al. Eur Heart J 2011; Wright RS et al. J Am Coll Cardiol 2011]. This article discusses selecting the appropriate therapies and improving short- and long-term outcomes in patients with NSTE-ACS.
- Coronary Artery Disease
- Myocardial Infarction
- Coronary Artery Disease
- Myocardial Infarction
- Cardiology
Patients with non-ST-elevation (NSTE) acute coronary syndrome (ACS) are treated with antiplatelet and anticoagulation to reduce ischemic complications [Hamm CW et al. Eur Heart J 2011; Wright RS et al. J Am Coll Cardiol 2011]. The presentations in this session focused on selecting the appropriate therapies and improving short- and long-term outcomes in patients with NSTE-ACS.
CHOOSING THE BEST ANTITHROMBIN THERAPY
Marco Valgimigli, MD, PhD, Erasmus Medical Center, Rotterdam, The Netherlands, reviewed the evidence on anticoagulants for patients with NSTE-ACS. The anticoagulants, unfractionated heparin (UFH), enoxaparin, and fondaparinux are recommended for patients with NSTE-ACS regardless of whether an early invasive or conservative strategy is chosen. In constrast, bivalirudin is only indicated for patients who are being considered for revascularization with an early invasive strategy [Hamm CW et al. Eur Heart J 2011; Wright RS et al. J Am Coll Cardiol 2011].
A meta-analysis of six trials reported an initial 50% relative risk reduction (RRR) in myocardial infarction (MI) or death and a 2-fold increase in major bleeding in patients treated with UFH plus acetylsalicylic acid (ASA) versus ASA alone. After 12 weeks the RRR decreased to 33% [Oler A et al. JAMA 1996]. Results of other UFH studies are shown in Table 1.
Studies of Unfractionated Heparin
In OASIS-5 [Yusuf S et al. N Engl J Med 2006], fondaparinux was noninferior to enoxaparin for reducing ischemic events at 9 days but significantly reduced mortality by 17% (HR, 0.83; 95% CI, 0.71 to 0.97; p=0.02) and bleeding by 50% (HR, 0.52; 95% CI, 0.44 to 0.61; p<0.001).
The ACUITY trial [Stone GW et al. N Engl J Med 2006] reported that for patients receiving percutaneous coronary intervention (PCI), bivalirudin plus provisional glycoprotein IIb/IIIa inhibitor (GPI) was noninferior to UFH or enoxaparin ± provisional GPI for the ischemic events while major bleeding significantly lower (3.0% vs 5.7%; RR, 0.53; 95% CI, 0.43 to 0.65; p<0.001). In ISAR-REACT 4, [Kastrati A et al. N Engl J Med 2011] patients with NSTE-ACS treated with PCI were randomized to bivalirudin or UFH plus abciximab. Bivalirudin was noninferior for the endpoint of death, recurrent MI or urgent target vessel revascularization (13.4% vs 12.8%; RR, 0.96; 95% CI, 0.74 to 1.25; p=0.76) and had lower rates of major bleeding (2.6% vs 4.6%; RR, 1.84; 95% CI, 1.10 to 3.07; p=0.02).
Prof. Valgimigli concluded that fondaparinux in medically treated patients and bivalirudin in PCI patients appears to be efficacious while having a favorable safety profile in terms of bleeding.
CHOOSING THE RIGHT ANTIPLATELET AGENT
The current antiplatelet agents available for P2Y12 inhibition in patients with NSTE-ACS are clopidogrel, prasugrel, and ticagrelor. Strategies to select the optimal agent were discussed by Matthew J. Price, MD, Scripps Clinic, La Jolla, California, USA.
Table 2 summarizes major trials evaluating antiplatelet agents in patients with ACS. These studies demonstrate the efficacy of prasugrel and ticagrelor compared with clopidogrel, although these agents also were associated with higher bleeding rates.
Antiplatelet Studies in NSTE-ACS
Dr. Price concluded that treatment for NSTE-ACS should be individualized according to ischemic and bleeding risk. Prasugrel or ticagrelor are preferred when markers of ischemic risk are present, including positive troponin or elevated biomarkers correlated with higher absolute event rates. For noninvasively managed patients, ticagrelor is superior to clopidogrel. Clopidogrel should be used when bleeding risk is high, particularly when concomitant oral anticoagulation is indicated or contraindications are present.
NOVEL ANTICOAGULATION TARGETS AND AGENTS
According to John H. Alexander, MD, MHS, Duke Clinical Research Institute, Durham, North Carolina, USA, available anticoagulants are effective for NSTE-ACS, but there is room for improvement both for control of ischemic events and for bleeding complications.
Otamixaban is a specific, direct Factor Xa inhibitor that was studied in moderate- to high-risk NSTE-ACS with planned early invasive management [Steg PG et al. JAMA 2013]. Compared to UFH plus eptifibatide, otamixaban did not improve rates of death or MI (5.5% vs 5.7%; RR, 0.99; 95% CI, 0.85 to 1.16; p=0.93) and increased major bleeding (3.1% vs 1.5%; RR, 2.13; 95% CI, 1.63 to 2.78; p<0.001).
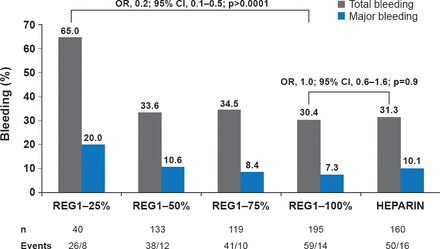
REG1 is a two-component, actively controllable anticoagulant system consisting of the Factor IX inhibitor, pegnivacogin and the control agent, anivamerson, which has a specific affinity for pegnivacogin. The RADAR trial [Povsic TJ et al. Eur Heart J 2013] evaluated REG1 versus heparin in NSTE-ACS patients undergoing femoral catheterization. At 30 days, ACUITY bleeding had occurred in 65.0%, 33.6%, 34.5%, 30.4%, and 31.3% of patients with 25%, 50%, 75%, and 100% reversal and heparin, respectively (Figure 1). At least 50% pegnivacogin reversal was needed to prevent bleeding. There were numerically but not statistically fewer ischemic events with REG1 versus heparin (3.0% vs. 5.7%, p=0.1). A Phase 3 trial comparing REG1 with bivalirudin, REGULATE-PCI, is ongoing [NCT01848106].
RADAR: ACUITY Bleeding Through 30 Days
REG1=pegnivacogin plus control agent, anivamerson.
Reproduced from Povsic TJ et al. A Phase 2, randomized, partially blinded, active-controlled study assessing the efficacy and safety of variable anticoagulation reversal using the REG1 system in patients with acute coronary syndromes: results of the RADAR trial. Eur Heart J 2013;34(31)248–2489. With permission from Oxford University Press.
Post-ACS, a number of new oral anticoagulants (NOACs) have been studied. A meta-analysis of the NOACs, ximeligatran, apixaban, rivaroxaban, dabigatran, and darexaban added to single or dual antiplatelet therapy (DAPT) for post-ACS therapy, reported only modest reductions in cardiovascular events and substantial increases in bleeding [Oldgren J et al. Eur Heart J 2013]. Included studies included a variety of agents as well as intensities of anticoagulation.
Dr. Alexander concluded that available anticoagulants are effective for acute management of NSTE-ACS but challenges remain with ischemic events and bleeding. For post-acute management of NSTE-ACS, adding an anticoagulant at therapeutic doses to current antiplatelet may reduce ischemic events and but increases bleeding. Future studies exploring different doses and new combinations of antiplatelet and anticoagulant agents will define the role of anticoagulation in post-ACS treatment.
LONG-TERM ANTICOAGULATION THERAPY
Freek W.A. Verheugt, MD, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands, explored the use of novel anticoagulants for long-term therapy and described the potential for eliminating aspirin or warfarin. Results of a nationwide cohort study in Denmark are shown in Table 3 [Lamberts M et al. Circulation 2012].
Bleeding and Ischemic Events
The ASPECT-2 [van Es RF et al. Lancet 2002] and WARIS-II [Hurlen M et al. N Engl J Med 2002] studies reported that warfarin alone or combined with aspirin was more effective for preventing ischemic events after MI than aspirin alone but was associated with increased bleeding. In the WOEST trial [Dewilde WJM et al. Lancet 2013], patients treated with OAC plus clopidogrel and ASA versus OAC plus clopidogrel had significantly increased bleeding (44.4% vs 19.4%; HR, 0.36; 95% CI, 0.26 to 0.50; p<0.0001) and all-cause mortality (6.3% vs 2.5%; HR, 0.39; 95% CI, 0.16 to 0.93; p=0.027). Ischemic events were not increased by dropping ASA. Another recent study found that OAC plus clopidogrel was equal to or better than triple therapy with respect to ischemic events and bleeding rates [Lamberts M et al. J Am Coll Cardiol 2013].
In the RE-LY study [Dans AL et al. Circulation 2013], patients receiving DAPT plus warfarin or dabigatran (110 or 150 mg) had the highest major bleeding rates compared with those receiving single or no antiplatelet therapy, with the lowest absolute risk among patients on dabigatran 110 mg.
The evidence shows that in patients requiring oral anticoagulation, DAPT reduces recurrent ischemic events after stenting for ACS but increases bleeding significantly. The use of safer OACs may reduce bleeding in this situation. Omitting ASA and treating with an anticoagulant and clopidogrel after stenting in atrial fibrillation patients seems promising but needs to be confirmed in future trials.
- © 2014 MD Conference Express®