Summary
In the first head-to-head randomized comparison of two devices used for transcatheter aortic valve replacement in high-risk patients with severe aortic stenosis, a balloon-expandable transcatheter valve was found to have a higher rate of device success than a self-expanding valve. This article discusses data from the Comparison of Transcatheter Heart Valves in High Risk Patients With Severe Aortic Stenosis: Medtronic CoreValve Versus Edwards SAPIEN XT trial [CHOICE; Abdel-Wahab M et al. JAMA 2014].
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Valvular Disease
- Cardiology & Cardiovascular Medicine
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Valvular Disease
In the first head-to-head randomized comparison of two devices used for transcatheter aortic valve replacement (TAVR) in high-risk patients with severe aortic stenosis (AS), a balloon-expandable transcatheter valve was found to have a higher rate of device success than a self-expanding valve.
Data from the Comparison of Transcatheter Heart Valves in High Risk Patients With Severe Aortic Stenosis: Medtronic CoreValve Versus Edwards SAPIEN XT trial [CHOICE; Abdel-Wahab M et al. JAMA 2014], were presented by Mohamed Abdel-Wahab, MD, Academic Teaching Hospital of the Universities of Kiel and Hamburg, Bad Segeberg, Germany.
The primary objective of CHOICE was to compare the procedural success of the two valves in patients with symptomatic severe AS who were at high surgical risk or deemed inoperable. Procedural success was defined as successful vascular access, deployment of the device, retrieval of the delivery system, correct position of the device, intended performance of the heart valve without moderate or severe regurgitation, and only one valve implanted in the proper anatomical location. The combined safety endpoint was a composite of all-cause mortality, major stroke, life-threatening or disabling bleeding, acute kidney injury Stage 3 (including renal replacement therapy), periprocedural myocardial infarction, major vascular complications and repeat procedure for valve-related dysfunction.
In five centers in Germany, 241 patients at high risk of surgical aortic valve replacement with suitable transfemoral vascular access were randomized to either the balloon-expanding valve (n=121) or the self-expanding valve group (n=120). Device size selection was based on manufacturers' sizing charts, but the study's steering committee strongly recommended sizing to be based on 3D imaging. All procedures were performed by experienced operators in centers with an established multidisciplinary TAVR program.
Following implantation, aortic insufficiency (AI) was assessed using angiography, transthoracic echocardiography, and invasive hemodynamic measurements. Valve function at follow-up was evaluated using transthoracic echocardiography and cardiac magnetic resonance imaging. Assessment of postprocedural AI utilized core laboratory angiography.
The average age of patients in the study was 80 years. Comorbidities, severity of AS and mean annulus diameter (measured with either transesophageal echocardiography or multislice computed tomography) were similar between the two groups. The most common valve size in the balloon-expandable arm was 26 mm and 29 mm in the self-expandable arm.
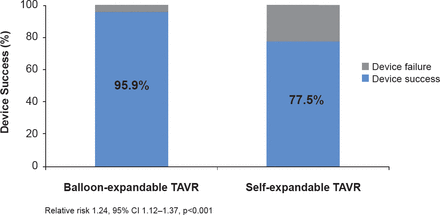
The occurrence of postprocedural AI on angiography (either any degree or greater than mild) was significantly less (p<0.001) in the balloon-expandable group. Patients in the balloon-expandable group underwent fewer procedures to reduce AI following valve implantation. Device success occurred in 95.9% of patients treated with the balloon-expandable device compared with 77.5% of patients in the self-expanding-device group (RR, 1.24; 95% CI, 1.12 to 1.37; p<0.001; Figure 1). This difference in device success in favor of the balloon-expandable device was attributed to the lower rate of moderate or severe AI in this group compared with the group treated with the self-expandable device (4.1% vs 18.3%; p<0.001), and the less frequent implantation of more than one valve (0.8% vs 5.8%; p=0.03).
Primary Endpoint
TAVR=transcatheter aortic valve replacement.
Reproduced with permission from M Abdel-Wahab, MD.
Clinical outcomes, including all-cause mortality and cardiovascular mortality at 30 days, were not significantly different between the groups (Table 1). The combined safety endpoint occurred in 18.2% in the balloon-expandable group and 23.1% in the self-expandable group. There was a numerical excess of stroke that did not reach statistical significance in the patients treated with balloon expandable valve (n=7) as compared with the patients treated with self-expandable valves (n=3). There were five rehospitalizations for heart failure in the self-expandable group and none in the balloon-expandable group. Patients in the balloon-expandable group required fewer new permanent pacemakers (17.3% vs 37.6%; p=0.001).
Clinical Outcomes at 30 Days
This investigator-initiated comparative effectiveness trial provides near-term outcomes in a head-to-head comparison of these alternative TAVR devices in experienced operator centers. Studies with larger samples sizes and longer follow-up are warranted to further evaluate the relative efficacy and safety of these TAVR platforms.
- © 2014 MD Conference Express®