Summary
Previous research suggests that catheter-based renal artery denervation (RDN) reduces blood pressure in patients with resistant hypertension. This article presents the results of the Renal Denervation in Patients With Uncontrolled Hypertension trial [SYMPLICITY HTN-3; Bhatt DL et al. N Engl J Med 2014]. This was a large, prospective, randomized, blinded sham-controlled study conducted to assess the safety and efficacy of percutaneous RDN in patients with resistant hypertension.
- Interventional Radiology
- Hypertension & Kidney Disease
- Renal Disease
- Hypertensive Disease
- Cardiology Clinical Trials
- Interventional Radiology
- Cardiology & Cardiovascular Medicine
- Hypertension & Kidney Disease
- Renal Disease
- Hypertensive Disease
- Cardiology Clinical Trials
Previous research suggests that catheter-based renal artery denervation (RDN) reduces blood pressure (BP) in patients with resistant hypertension. Deepak L. Bhatt, MD, MPH, Brigham and Women's Hospital, Boston, Massachusetts, USA, presented the results of the Renal Denervation in Patients With Uncontrolled Hypertension trial [SYMPLICITY HTN-3; Bhatt DL et al. N Engl J Med 2014]. This was a large, prospective, randomized, blinded sham-controlled study conducted to assess the safety and efficacy of percutaneous RDN in patients with resistant hypertension.
The study was conducted at 88 sites in the United States. Patients were eligible if they were aged 18 to 80 years at the time of randomization, were on a stable regimen of ≥3 antihypertensive medications of different classes (including a diuretic) with no changes in the previous 2 weeks and no expected changes in the next 6 months, and had a mean office systolic BP (SBP) ≥160 mm Hg. Patients with any of the following were excluded: an ambulatory BP monitor (ABPM) 24-hour average SBP <135 mm Hg, an estimated glomerular filtration rate of <45 mL/min/1.73 m2, main renal arteries <4 mm diameter or <20 mm treatable length, multiple renal arteries, renal artery stenosis >50%, an aneurysm in either renal artery, or a history of prior renal artery intervention.
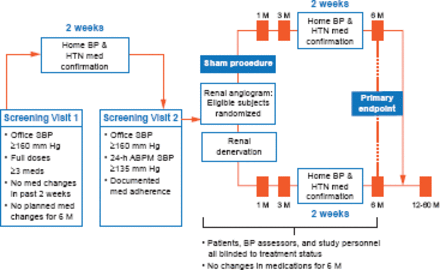
The study design is illustrated in Figure 1. Patients were randomized to either RDN or a sham procedure (renal angiogram). The primary endpoint was the change in SBP (office, superiority margin 5 mm Hg) at 6 months with the change in mean 24-hour ambulatory SBP as a secondary endpoint. The primary safety endpoint was a composite of death, end-stage renal disease, embolic events resulting in end-organ damage, renovascular complications, or hypertensive crisis at 1 month or new renal-artery stenosis at 6 months.
Study Schematic
ABPM=ambulatory blood pressure monitor; BP=blood pressure; HTN=hypertension; M=months; SBP=systolic blood pressure.
Reproduced from Kandzari DE et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol 2012; 35(9):528–535. With permission from John Wiley and Sons.
A total of 535 patients were randomized to RDN (n=364) and to the sham procedure (n=171). The mean age was 57 years, ∼62% were male, ∼70% were white, and patients were taking a mean of five antihypertensive agents. The mean baseline office SBP was 180 mm Hg, and the mean baseline ABPM was 160 mm Hg.
SBP in the two groups at 6 months was similar (difference of −2.39 mm Hg; 95% CI, −6.89 to 2.12; p=0.26) and SBP in both arms decreased from baseline to 6 months −14.1 mm Hg for the RDN group and −11.7 mm Hg for the sham group.
There was no difference in the major adverse event rate for RDN (1.4%) versus the sham arm (0.6%; p=0.67). The secondary efficacy analysis of the change in 24-hour mean systolic ABPM was also nonsignificant: −1.96 mm Hg (95% CI, −4.97 to 1.06).
When presenting these results Dr. Bhatt concluded, “Further study in rigorously designed clinical trials will be necessary to confirm previously reported benefits of renal denervation in patients with resistant hypertension or to validate alternate methods of renal denervation.”
- © 2014 MD Conference Express®