Summary
The Clinical Trial Comparing Cangrelor to Clopidogrel Standard Therapy in Subjects Who Require Percutaneous Coronary Intervention [CHAMPION PHOENIX; NCT01156571] randomized 11,145 patients undergoing percutaneous intervention in a double-blind, double-dummy fashion to a bolus and infusion of cangrelor followed by clopidogrel or to clopidogrel 600 or 300 mg. This updated analysis examines the robustness of the effect of cangrelor on ischemic events using sequential sensitivity analyses of the primary composite end point by excluding stent thrombosis events that occurred during the procedure and applying increasingly stringent criteria for myocardial infarction.
- Myocardial Infarction
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Myocardial Infarction
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Cardiology
The Clinical Trial Comparing Cangrelor to Clopidogrel Standard Therapy in Subjects Who Require Percutaneous Coronary Intervention [CHAMPION PHOENIX; NCT011 56571] randomized 11,145 patients undergoing percutaneous intervention (PCI) in a double-blind, double-dummy fashion to a bolus and infusion of cangrelor followed by clopidogrel or to clopidogrel 600 or 300 mg. The investigators reported significant reductions in the composite end point of death, myocardial infarction (MI), ischemia-driven revascularization (IDR), and stent thrombosis (ST) in patients treated with cangrelor vs clopidogrel (4.7% vs 5.9%; P = .005) [Bhatt DL et al. N Engl J Med 2013].
The objective of this analysis of the CHAMPION PHOENIX trial, presented by Deepak L. Bhatt, MD, MPH, Brigham and Women's Hospital, Boston, Massachusetts, USA, was to examine the robustness of the effect of cangrelor on ischemic events using sequential sensitivity analyses of the primary composite end point by excluding stent thrombosis events that occurred during the procedure (IPST) and applying increasingly stringent criteria for MI.
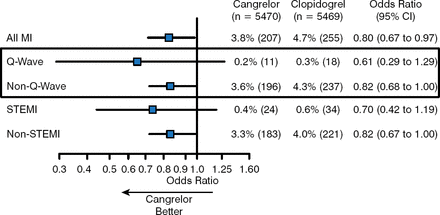
In an analysis of its effect on MI stratified by type at 48 hours, cangrelor vs clopidogrel reduced the rates of all MI (3.8% vs 4.7%; OR 0.80; 95% CI, 0.67 to 0.97); Q-wave MI (0.2% vs 0.3%; OR 0.61; 95% CI, 0.29 to 1.29); non-Q-wave MI (3.6% vs 4.3%; OR 0.82; 95% CI, 0.68 to 1.00); STEMI (0.4% vs 0.6%; OR 0.70; 95% CI, 0.42 to 1.19); and non-STEMI (3.3% vs 4.0%; OR 0.82; 95% CI, 0.67 to 1.00; Figure 1) [Cavender M et al. Eur Heart J 2014 (abstr P732)].
Effect of Cangrelor on Myocardial Infarction at 48 Hours Stratified by Type
MI, myocardial infarction.
Reproduced with permission from M Cavender, MD.
Cangrelor, when compared with clopidogrel, reduced the rates of all type 4a MI stratified by peak creatine kinase MB (CK-MB) at 48 hours: CK-MB ≥ 3x upper limit of normal (ULN; 3.5% vs 4.4%; OR 0.80; 95% CI, 0.66 to 0.98); CK-MB ≥ 5x ULN (1.9% vs 2.4%; OR 0.82; 95% CI, 0.63 to 1.06); CK-MB ≥ 10x ULN (0.7% vs 1.1%; OR 0.59; 95% CI, 0.39 to 0.89).
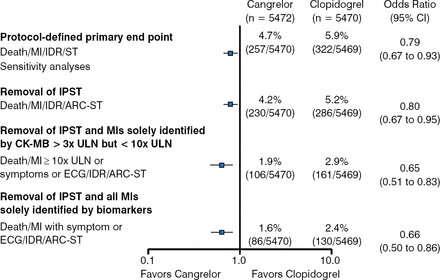
The results of sensitivity analyses that removed components of the primary end point showed that cangrelor continued to show efficacy even with the removal of IPST (4.2% vs 5.2%; OR 0.80; 95% CI, 0.67 to 0.95). Similar results were found after the removal of IPST and MI identified solely by CK-MB with peak cardiac biomarkers > 3x ULN and < 10x ULN (1.9% vs 2.9%; OR 0.65; 95% CI, 0.51 to 0.83); and removal of IPST and MI identified solely by biomarkers (1.6% vs 2.4%; OR 0.66; 95% CI, 0.50 to 0.86; Figure 2).
Sensitivity Analyses for Composite End Points
ARC-ST, Academic Research Consortium stent thrombosis; CK-MB, creatine kinase MB; ECG, electrocardiography; IDR, ischemia-driven revascularization; IPST, intraprocedural stent thrombosis; MI, myocardial infarction; ST, stent thrombosis; ULN, upper limit of normal.
Reproduced with permission from DL Bhatt, MD.
Additional sensitivity analyses showed that cangrelor vs clopidogrel reduced the risk of the following composite end points: death, MI with peak rise in cardiac biomarkers ≥ 10x ULN, IDR, or Academic Research Consortium (ARC)-ST (1.4% vs 2.0%; OR 0.69; 95% CI, 0.51 to 0.92); death, MI with peak rise in cardiac biomarkers ≥ 10x ULN in patients with cardiac biomarkers at baseline, IDR, or ARC-ST (1.4% vs 2.0%; OR 0.69; 95% CI, 0.51 to 0.93); and death, Q-wave MI, IDR, or ARC-ST (0.9% vs 1.2%; OR 0.76; 95% CI, 0.53 to 1.11).
These analyses demonstrated consistent risk reduction with cangrelor across multiple end points. Even when all biomarker-defined MIs were excluded, the risk reduction of cangrelor remained significant. Thus, cangrelor reduced important ischemic events, even when using the most restrictive definitions of MI and ST when compared with clopidogrel. Dr Bhatt concluded that cangrelor is superior to clopidogrel for reducing important clinical events in patients undergoing PCI.
- © 2014 MD Conference Express®