Summary
Atrial fibrillation (AF) is a worldwide public health problem, with a prevalence of 10% to 15% after 80 years of age. The morbidity and mortality secondary to AF is high. With regard to the outcomes of stroke, the attributable risk of AF increases sharply from 1.5% to 23.5% for ages 50 to 59 years and 80 to 89 years, respectively. AF-related stroke is particularly concerning as it carries a high mortality, with 1 in 4 patients dying within 30 days. This article discusses stroke prevention in elderly patients with AF, including data on the advances in pharmacological therapeutic and agent selection that are used to balance thromboembolic risk with bleeding risk in patients with AF.
- Thrombotic Disorders
- Cerebrovascular Disease
- Prevention & Screening
- Arrhythmias
- Cardiology
- Thrombotic Disorders
- Cerebrovascular Disease
- Prevention & Screening
- Arrhythmias
Atrial fibrillation (AF) is a worldwide public health problem, with a prevalence of 10% to 15% after 80 years of age. The morbidity and mortality secondary to AF is high. With regard to the outcomes of stroke, the attributable risk of AF increases sharply from 1.5% to 23.5% for ages 50 to 59 years and 80 to 89 years, respectively. AF-related stroke is particularly concerning as it carries a high mortality, with 1 in 4 patients dying within 30 days. Much of AF may be caused by the underlying processes contributing to aging of the vasculature, including a decreased ability to manage the oxidative stress caused by pro-inflammatory insults. Many of the risk factors that predict stroke in these patients also predict bleeding when antiplatelet agents or anticoagulants (AC) are used.
In her State of the Art lecture on preventing stroke in elderly patients with AF, Elaine M. Hylek, MD, Boston University Medical Center, Boston, Massachusetts, USA, discussed data on the advances in pharmacological therapeutic and agent selection that are used to balance thromboembolic risk with bleeding risk in patients with AF. Beyond appropriate drug selection, treatment barriers, such as gender bias, adherence factors (eg, perception of bleeding risk) and polypharmacy, were reviewed. Other strategies to reduce stroke, such LAA occlusion, were also discussed. Finally, she highlighted the need for the prevention of AF by treatment of its underlying causes, including obesity.
Undertreatment of thromboembolic risk in patients with AF has been demonstrated. In an analysis of primary care practices in the UK [Freedman SB et al. Eur Heart J. 2014 (abstr 4183)], 30% of all patients with AF were being treated with only an antiplatelet (AP) drug, despite a mean CHA2DS2-VASc score of 2.9. The data also illustrated a persistent gender bias, as seen across the spectrum of cardiovascular disease (CVD), with 43% of women receiving only an AP drug. The international GARFIELD and GLORIA-AF registries have shown that utilization of AC therapy does not increase with higher CHADS2 scores, demonstrating that those with the highest stroke risk are inadequately treated [Kakkar AJ et al. PLoS One. 2013 and Huisman MV et al. Eur Heart J. 2014 (abstr P4394)]. Dr Hylek stated these data reinforce the need for better education of physicians and patients and the need to identify healthcare system barriers to adequate anticoagulation in certain countries.
Aspirin should not have a significant role in the prevention of AF-related stroke, stated Dr Hylek. The BAFTA study [Mant J et al. Lancet. 2007] showed in patients aged > 75 years that warfarin reduced the risk of fatal or nonfatal disabling stroke or significant arterial embolism by 47% as compared with aspirin (HR, 0.48; 95% CI, 0.28 to 0.80). The reduced efficacy of aspirin was not accompanied by a more benign bleeding profile (as commonly believed); the bleeding risk was similar between warfarin and aspirin for major extracranial hemorrhages (HR, 0.87; 95% CI, 0.43 to 1.73) and all major hemorrhages (HR, 0.96; 95% CI, 0.53 to 1.75). Supporting data from a meta-analysis showed a 64% versus 22% risk reduction in stroke with adjusted-dose warfarin as compared to AP [Hart RG et al. Ann Intern Med. 2007]. The AVERROES trial demonstrated that the rates of major bleeding are similar between apixiban and aspirin (RR, 1.18; 95% CI, 0.77 to 1.80; P = .76) [Connolly SJ, et al. N Engl J Med. 2011], again suggesting no significant safety benefit of aspirin over AC, especially in an older population.
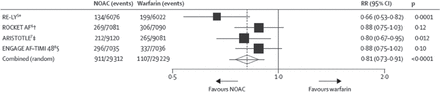
Novel oral anticoagulants (NOACs) provided a greater reduction in the combined end point of ischemic and hemorrhagic stroke as compared with warfarin, according to a meta-analysis of 4 phase 3 trials (Figure 1) [Ruff CT et al. Lancet. 2014]. Importantly, NOACs reduce the risk of intracerebral hemorrhage (ICH) by 60% compared to vitamin K antagonists [Debette S, Markus HS. BMJ. 2010], possibly due to a lack of Factor VII suppression. ICH is a rare (approximately 1 in 250 patients), but it is a devastating complication of AC therapy, often stemming from the increase in white matter disease with age, hypertension, diabetes, and amyloidosis.
Efficacy of NOACS Versus Warfarin in Clinical Trials
On December 11, 2014, this figure was changed to the correct figure. Data are n/N, unless otherwise indicated. Heterogeneity: I 2 = 47%; P = .13. NOAC, novel oral anticoagulant. *Dabigatran 150 mg twice daily. † Rivaroxaban 20 mg once daily. ‡ Apixaban 5 mg twice daily. ∫ Edoxaban 60 mg once daily.
Reprinted from Ruff CT et al, Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. The Lancet. 2014;383:955–962. Copyright © 2014, with permission from Elsevier.
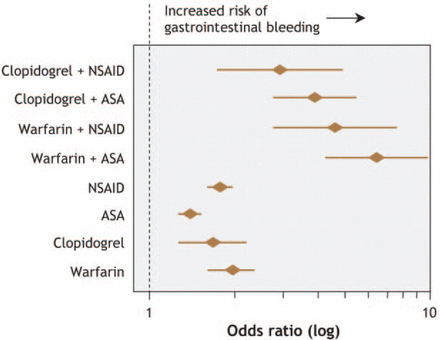
Although the overall risk of major bleeding was reduced by 14% with NOACS as compared warfarin for the combined trials in this meta-analysis, gastrointestinal (GI) bleeding remains a prominent concern, especially for an elderly population. The incidence of upper and lower GI bleeding increases with age, with 70% of acute upper GI bleeding occurring in persons aged > 60 years. The reasons for this high risk of GI bleeding in older patients includes concomitant aspirin use; changes in GI physiology, such as luminal effects, mucosal protection, and reparative capacity [Newton JL. Clin Interv Aging. 2006]; and a prevalence of Helicobacter pylori in up to 80% of patients aged > 80 years in some countries. Dr Hylek stated that these factors help to explain some of the difficulty in maintaining older patients on an AC. The added risk of GI bleeding with concomitant AP, warfarin, and nonsteroidal anti-inflammatory drugs (NSAIDs) is shown in Figure 2 [Delaney JA et al. CMAJ. 2007]. The addition of AP or NSAIDs to a treatment regimen that included a NOAC would also likely increase the risk of GI bleeding, noted Dr Hylek.
Risk of GI Bleeding With Antithrombotics or NSAIDs
ASA, aspirin; NSAID, nonsteroidal anti-inflammatory drug.
Reproduced from Delaney JA et al. Drug—drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ. 2007;177:347–51. With permission from Canadian Medical Association.
Following a GI bleed, re-challenging patients with AC therapy is often difficult. The need to resume treatment is supported by a study demonstrating that, in patients with a prior GI bleed, the resumption of warfarin led to a reduction in thrombotic events (HR, 0.05; 95% CI; 0.01 to 0.58) and death (HR, 0.31; 95% CI, 0.15 to 0.62), and was not accompanied by a significant increase in recurrent GI bleeding (HR, 1.32; 95% CI, 0.50 to 3.57) [Witt DM et al. Arch Intern Med. 2012].
An adherence to AC may be compromised by polypharmacy, with the number of drugs being the strongest predictor of nonadherence. About 75% of patient nonadherence is intentional to decrease the burden of taking pills, reduce adverse effects, or decrease costs [Wu JY et al. BMJ. 2006]. One study showed that persistence in taking a vitamin K antagonist was only about 30% at 360 days [Simonyi G, Molnar MP. ESC 2014; (abstr 4184)].
Intervention-based strategies that are being developed to prevent stroke in AF include catheter-based, left atrial appendage closure [Tzikas A, et al. ESC 2014; (abstr 4185)] as well as possible pharmaceutical strategies, which include different targets for thrombus that result in reduced bleeding. A recent study showed that paroxysmal AF is associated with a lower level of risk for stroke versus persistent or permanent AF [Vanassche T, et al. ESC 2014; (abstr 4182)], and this may provide some clues for new approaches, stated Dr Hylek.
The prevention of AF may be the most important goal if the burden of AF is to be reduced. A recent study demonstrated a linear increase between the CHA2DS2-VASc score and the risk of new AF, underscoring the effect of the aging vasculature on AF risk [Fauchier L et al. Eur Heart J. 2014 (abstr P4229)]. It is possible to reduce the burden and severity of AF by addressing obesity and other cardiometabolic risk factors [Abed HS et al. JAMA. 2013]. It is likely that much of the improvement is mediated by a reduction in inflammation; one study showed that a decrease in body mass index was associated with significant reductions in C-reactive protein and AF burden and symptoms. Scientific work is exploring the link between inflammation and thrombosis. It has been shown that, in the presence of inflammation, there is an upregulation in genes that stimulate the coagulation cascade. Additionally, investigators have shown that neutrophils activation results in a release of myeloperoxidase, which in turn leads to endothelial damage, increased fibrosis, and the development of tissue that is conducive to AF. MR imaging has demonstrated that left atrial fibrosis is associated with an increased risk of stroke in patients with AF and with recurrent AF after ablation [Daccarett M et al. J Am Coll Cardiol. 2011]. The different stages of fibrosis may provide an opportunity to disrupt these pathways, alter the substrate, and prevent or reverse fibrosis.
In addressing obesity and other cardiometabolic risk factors, it is possible to reduce the burden and severity of AF [Abed HS et al. JAMA. 2013] by reducing inflammation caused by the presence of these risk factors, stated Dr Hylek. Although these findings await further validation, Dr Hylek stated that weight reduction strategies should be part of the initial education that is provided by cardiovascular intervention laboratories.
The editors would like to thank the many members of the 2014 European Society of Cardiology presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2014 MD Conference Express®