Summary
The Stability of Atherosclerotic Plaque by Initiation of Darapladib Therapy trial [STABILITY; NCT00799903] showed that darapladib compared with placebo, did not significantly reduce the composite primary end point of cardiovascular death, myocardial infarction, and stroke in 15?828 patients with stable coronary heart disease on optimal medical therapy [White HD et al. N Engl J Med. 2014]. This article presents data from a predefined analysis of STABILITY designed to examine factors that contribute to high levels of Lp-PLA2 activity, whether its activity predicted outcomes or identified patients who would have a greater benefit with darapladib, and whether darapladib would provide persistent, long-term reduction of Lp-PLA2 activity.
- Cardiology Clinical Trials
- Coronary Artery Disease
- Cardiology
- Cardiology Clinical Trials
- Coronary Artery Disease
The Stability of Atherosclerotic Plaque by Initiation of Darapladib Therapy trial [STABILITY; NCT00799903] showed that darapladib, an oral inhibitor of lipoprotein-associated phospholipase A2 (Lp-PLA2), compared with placebo, did not significantly reduce the composite primary end point of cardiovascular (CV) death, myocardial infarction (MI), and stroke (HR, 0.94; 95% CI, 0.85 to 1.03; P = .20) in 15 828 patients with stable coronary heart disease (CHD) on optimal medical therapy [White HD et al. N Engl J Med. 2014]. The mean follow-up duration was 3.7 years. The secondary end point of major coronary events (CHD death, MI, and urgent coronary revascularization) was significantly reduced with treatment versus placebo (HR, 0.90; 95% CI, 0.82 to 1.00; P = .045).
Lars Wallentin, MD, PhD, Uppsala Clinical Research Center, Uppsala, Sweden, presented data from a predefined analysis of STABILITY designed to examine factors that contribute to high levels of Lp-PLA2 activity, whether its activity predicted outcomes or identified patients who would have a greater benefit with darapladib, and whether darapladib would provide persistent, long-term reduction of Lp-PLA2 activity.
For this analysis, blood samples were collected at baseline from 14 500 patients; of these, samples were also collected at all follow-up visits (months 1, 3, 6, and 18 and end of treatment) in 100 patients. The mean activity level of Lp-PLA2 was 172 nmol/min/mL, with a normal distribution in relation to baseline demographics. The patients were evenly divided by tertiles of Lp-PLA2 activity (tertile 1, ≥ 153.6 nmol/min/mL; tertile 2, 153.7–192.5 nmol/min/mL; tertile 3, > 192.5 nmol/min/mL).
The baseline characteristics and biomarkers that increased or decreased Lp-PLA2 activity on multivariate analysis are shown in Table 1. In the subset of 100 patients, there was a 65% relative risk reduction in Lp-PLA2 activity with darapladib that was seen 1 month after treatment began and persisted through the end of follow-up.
Effect of Baseline Characteristics and Biomarkers on Lp-PLA2 Activity on Multivariate Analysis
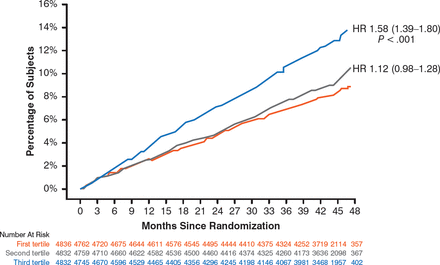
For the primary end point, the baseline level of Lp-PLA2 activity predicted an increased risk for events, with a significant increase in tertile 3 versus tertile 1 (P < .001) and a nonsignificant (NS) increase in tertile 2 versus tertile 1 (Figure 1). A similar prognostic effect was seen for the secondary end point of major coronary events for tertile 3 versus tertile 1 (HR, 1.52; 95% CI, 1.34 to 1.73; P < .001) and tertile 2 versus tertile 1 (HR, 1.12; 95% CI, 0.97 to 1.28; P = NS).
Lp-PLA2 Activity in Relation to Primary Outcome
C V, card iovascu la r; Lp-PL A2, lipoprotein-associated phospholipase A2; MI, myocardial infarction.
Reproduced with permission from L Wallentin, MD, PhD.
No relation was found between treatment with darapladib and the composite primary or secondary outcomes in any tertile of Lp-PLA2 activity (Table 2).
Treatment Outcomes in Relation to Lp-PLA2 Activity
In addition to traditional risk factors that increased the risk for a primary outcome event, Lp-PLA2 activity increased this risk (tertile 3 vs tertile 1; HR, 1.45; 95% CI, 1.25 to 1.69; P < .001). Darapladib versus placebo treatment did not influence the risk for the primary outcome (HR, 0.92; 95% CI, 0.83 to 1.03; P = .14).
This analysis of the STABILITY study showed that Lp-PLA2 activity is an independent predictor of CV events, but its baseline activity did not predict the effect of darapladib on coronary events.
Further evaluation is needed to determine the value of measuring Lp-PLA2 activity to predict CV risk in the absence of an indication for a specific treatment, stated Prof Wallentin.
- © 2014 MD Conference Express®