Summary
This article presents results from the Integrated Biomarker and Imaging Study-4 [IBIS-4; Räber L et al. Eur Heart J. 2014], a prospective substudy embedded in the COMFORTABLE trial [Räber L et al. JAMA. 2012] comparing biolimus-eluting stents vs bare-metal stents in patients with STEMI undergoing percutaneous coronary intervention (PCI).
- Interventional Techniques & Devices
- Cardiology
- Cardiac Imaging Techniques
- Coronary Artery Disease
- Imaging Modalities
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Cardiac Imaging Techniques
- Coronary Artery Disease
- Imaging Modalities
- Cardiology Clinical Trials
Lorenz Räber, MD, Bern University Hospital, Bern, Switzerland, presented results from the Integrated Biomarker and Imaging Study-4 [IBIS-4; Räber L et al. Eur Heart J. 2014], a prospective substudy embedded in the COMFORTABLE trial [Räber L et al. JAMA. 2012] comparing biolimuseluting stents vs bare-metal stents in patients with STEMI undergoing percutaneous coronary intervention (PCI). Data from IBIS-4 demonstrated that high-dose daily rosuvastatin was associated with a significant reduction in atherosclerotic burden in the non—infarct-related epicardial coronary arteries (non-IRAs) in patients with STEMI who underwent successful primary PCI.
For the past 2 decades, statins have been the mainstay of therapy in patients with high levels of low-density lipoprotein cholesterol (LDL-C), potently reducing cardiovascular events with acute coronary syndromes [Roth EM, Diller P. Future Cardiol. 2014]. According to Prof Räber, however, although statins are a key component of treatment for patients with acute STEMI, their long-term impact on coronary atherosclerosis is unknown. This study therefore aimed to investigate the effect of high-dose statin therapy on plaque burden, composition, and phenotype in the non-IRAs of patients with STEMI undergoing primary PCI.
IBIS-4 included 103 patients with STEMI who underwent intravascular ultrasonography (IVUS) and radiofrequency ultrasonography (RF-IVUS) of the 2 non-IRAs following successful primary PCI. Exclusion criteria included subjects with either non-IRA with > 50% stenosis.
All patients received rosuvastatin 20 mg/d for the first 2 weeks, followed by a dose increase to 40 mg/d for the remainder of the study period. Atherosclerotic burden was evaluated in the proximal arterial segments at baseline and 13 months using IVUS and RF-IVUS.
The primary IVUS end point was the change in percent atheroma volume (PAV), and the primary RF-IVUS end point was the change in percent necrotic core, both at 13 months. Successful serial imaging was available for 82 patients with 146 analyzed non-IRAs at both time points.
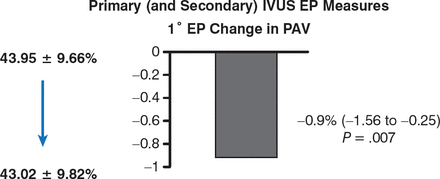
From baseline to 13 months, LDL-C had decreased from a median of 3.29 to 1.89 mmol/L (P < .001). With regard to the primary end point, IVUS demonstrated a significant reduction of atheroma volume (43.95% to 43.02%; 95% CI, −1.56 to −0.25, P = .007; Figure 1).
Effect of Rosuvastatin on Atheroma Volume
EP, end point; IVUS, intravascular ultrasonography; PAV, percent atheroma volume.
Source: Räber L et al. Eur Heart J. 2014.
Reproduced with permission from L Räber, MD.
There was no significant change, however, in percent necrotic core with RF-IVUS (21.14% to 21.02%; 95% CI, −1.05 to 0.96; P = .93). Similarly, the proportions of plaques with necrotic core and different plaque phenotypes were unchanged.
Dr Räber noted that the proximal segments of non-IRAs in these patients contained a high atherosclerotic plaque burden, with the majority of lesions comprising thin-cap fibroatheromas. In his concluding remarks, he also emphasized that although high-dose rosuvastatin therapy for 13 months is associated with a significant reduction of coronary atherosclerosis, it did not change the proportion of necrotic core in these arteries or the plaque phenotypes.
- © 2014 MD Conference Express®